This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Percutaneous dilatational tracheostomy (PDT) is widely used in intensive care units, but this conventional method has some disadvantages, such as requirement of a lot of equipment and experts at the site. Especially, in situations where the patient is isolated due to an infectious disease, difficulties in using the equipment may occur, and the number of exposed persons may increase. In this paper, we introduce hybrid tracheostomy that combines the advantages of surgical tracheostomy and PDT and describe our experiences.
Methods
Data from 55 patients who received hybrid tracheostomy without bronchoscopy from January 2020 to February 2021 were collected and reviewed retrospectively. Hybrid tracheostomy was performed at the bedside by a single thoracic surgeon. The hybrid tracheostomy method was as follows: after the skin was incised and the trachea was exposed, only the extent of the endotracheal tube that could not be removed was withdrawn, and then tracheostomy was performed by the Seldinger method using a PDT kit.
Results
The average age was 66.5 years, and the proportion of men was 69.1%. Among the patients, 21.8% were taking antiplatelet drugs and 14.5% were taking anticoagulants. The average duration of the procedure was 13.3 minutes. There was no major bleeding, and there was one case of paratracheal placement of the tracheostomy tube.
Conclusions
In most patients, the procedure can be safely performed without any major complications. However, patients with a short neck, a neck burn or patients who have received radiation therapy to the neck should be treated with conventional methods.
Go to :

Keywords: anticoagulants, bronchoscopy, intensive care units, tracheostomy, ventilator weaning
INTRODUCTION
Tracheostomy is performed in a significant number of patients treated in the intensive care unit (ICU) for prolonged ventilation or airway maintenance. Ever since percutaneous dilatational tracheostomy (PDT) was first introduced by Ciaglia et al. [
1] in 1985, it has become a widely used treatment in ICU settings because it is easier than surgical tracheostomy (ST) and can be performed by the bedside. Bronchoscopy and ultrasound guided PDT are the most commonly used percutaneous techniques [
2]. PDT is limited by several contraindications, such as history of previous surgery, difficult neck anatomy, and coagulopathies [
3,
4]. However, in most other cases, PDT shortens the time interval between the decision about a tracheostomy and the actual procedure, and the complication rate of PDT is similar to that of ST [
4,
5].
However, the conventional PDT method using a bronchoscope has some disadvantages, such as requirement of a lot of equipment and experts at the site. Especially, in situations where the patient is isolated due to an infectious disease, difficulties in using the equipment may occur, and the number of exposed persons may increase. For this reason, methods to reduce equipment and manpower, such as PDT using a light wand, are being studied [
6,
7]. In this paper, we introduce hybrid tracheostomy that combines the advantages of PDT and ST based on our experiences.
Go to :

MATERIALS AND METHODS
This study was approved by the Institutional Review Board of Konyang University Hospital (IRB No. 2021-02-010), and the requirement for informed consent was waived due to the retrospective nature of the study. From January 2020 to February 2021, we performed hybrid tracheostomy without bronchoscopy on ICU patients who showed indications for tracheostomy. We collected data from a total of 61 patients who received hybrid tracheostomy and reviewed them retrospectively. We gleaned data, including age, sex, body mass index (BMI), complications, antiplatelets and anticoagulants use, blood test results on the day of the procedure, and the reason for tracheostomy. We investigated the procedure, tube size, and immediate complications that occurred on the day of the procedure, as well as data related to the procedure. The duration of the procedure was defined as the time from skin incision to cannulation. Immediate complications were subdivided into oozing at the tracheostomy site, major bleeding requiring blood transfusion or surgical treatment, paratracheal placement of the tracheostomy tube, and pneumothorax. Finally, as shown in
Figure 1, we analyzed the data of 55 patients, after excluding two patients with incorrect data and four patients who underwent the procedure with bronchoscopy out of the 61 patients who received hybrid tracheostomy.
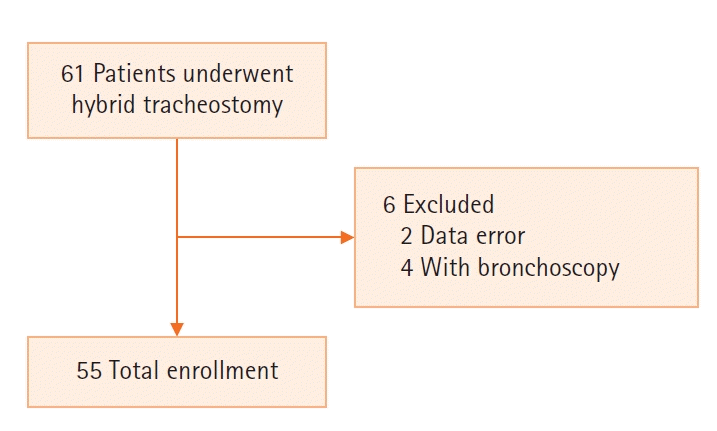 | Figure 1.Flowchart showing selection of study population. 
|
Hybrid tracheostomy was performed at the bedside by a single thoracic surgeon. Hybrid tracheostomy was performed in the following manner. After sedation and relaxation using midazolam and vecuronium, the patient's neck extension and surgical draping were performed, and then the skin was incised under local anesthesia. The skin was incised at about 1.0–2.0 cm below the cricoid cartilage in the transverse direction. After making the skin incision, the pretracheal tissue was dissected to expose the trachea. The tube was withdrawn only to the extent of the endotracheal tube that could not be removed. To determine the safe depth for withdrawing the endotracheal tube, we performed a pilot study using bronchoscopy. According to the results, tube withdrawal was safe to a depth of 16–18 cm at upper incisors. Hence, we withdrew the tube up to a depth of 16 cm at upper incisors. No desaturation event occurred. After that, tracheostomy was performed using the PDT kit (Ciaglia Blue Rhino Percutaneous Tracheostomy Introducer Kit; Cook Critical Care, Bloomington, IL, USA) while visually checking the exposed trachea (
Figure 2). After all procedures were completed, chest X-rays were taken to check for complications such as pneumothorax. There was one case of traumatic C-spine injury. Hence, in that case, we performed hybrid tracheostomy without neck extension.
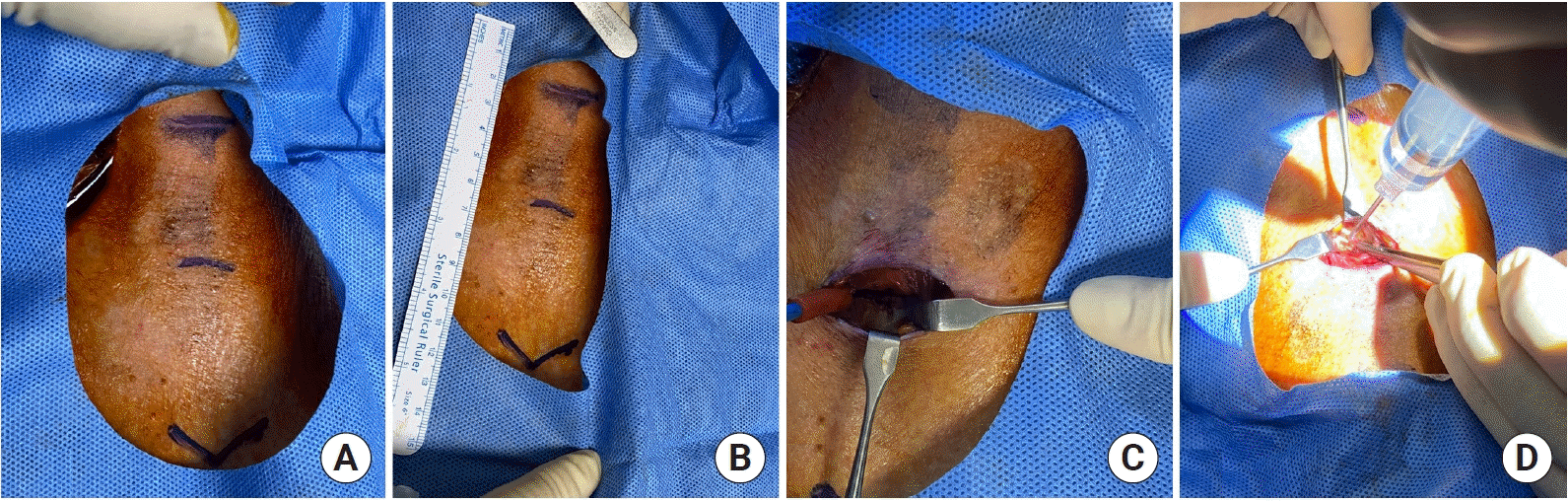 | Figure 2.Procedure photo. (A, B) Anatomical position indications. From above, thyroid cartilage, between the 2nd and 3rd tracheal rings, and sternal notch. (C) Exposed trachea by dissecting the pretracheal tissue after skin incision. (D) Photo of performing percutaneous dilatational tracheostomy while visually checking the exposed trachea. 
|
Go to :

RESULTS
A total of 55 patients were sampled, and their baseline characteristics are specified in
Table 1. The age of the patients ranged from 18 to 91 years, and the average age was 66.5 years. The male to female ratio was 38 (69.1%) to 17 (30.9%), and the average BMI was 21.8 kg/m
2. The comorbidities of patients were hypertension (34.5%), diabetes mellitus (21.8%), solid cancer (14.5%), and chronic lung disease (i.e., asthma, chronic obstructive pulmonary disease, and interstitial lung disease; 10.9%). The percentage of patients using antiplatelet drugs was 21.8% and the percentage of those using anticoagulants was 14.5%. None of the patients were taking both antiplatelets and anticoagulant drugs at the same time. Of the patients taking antiplatelet drugs, about a quarter of the patients were using double antiplatelet drugs. The most common reason for admission to the ICU was septic shock (49.1%), followed by respiratory failure excluding respiratory failure due to septic shock (23.6%). Neurological problems leading to ICU admissions included hemorrhage, cerebral infarction, and traumatic C-spine injury. Other reasons include intoxication (herbicides, alcohol), drowning, hanging, and hypovolemic shock due to variable bleeding. A total of 20% of patients underwent tracheotomy to maintain the airways, and 80% of patients underwent tracheotomy due to prolonged mechanical ventilation.
Table 1.
|
Variable |
Value (n=55) |
|
Age (yr) |
66.5 (18.0–91.0) |
|
Male |
38 (69.1) |
|
Body mass index (kg/m2) |
21.8 (15.6–33.3) |
|
Comorbidity |
|
|
Hypertension |
19 (34.5) |
|
Diabetes mellitus |
12 (21.8) |
|
Malignancy, solid |
8 (14.5) |
|
Chronic lung diseasea
|
6 (10.9) |
|
Use of antiplatelet agent |
12 (21.8) |
|
Single agent |
9 (16.4) |
|
Dual agents |
3 (5.5) |
|
Use of anticoagulation agent |
8 (14.5) |
|
Low molecular weight heparin |
2 (3.6) |
|
Direct oral anticoagulants |
1 (1.8) |
|
Nafamostat |
5 (9.1) |
|
Lab results on the day of procedure |
|
|
Hb (g/dl) |
9.96 (7.5–14.9) |
|
HCT (%) |
30.6 (23.2–45.7) |
|
PLT (×103/mm3) |
209.4 (37.0–584.0) |
|
PT (sec) |
14.4 (10.9–23.6) |
|
PT-INR |
1.28 (1.00–2.08) |
|
aPTT (sec) |
32.7 (23.9–53.6) |
|
Reason for admission to intensive care unit |
|
|
Acute cardiac event |
2 (3.6) |
|
Sepsis or septic shock |
27 (49.1) |
|
Respiratory failure (excluding sepsis/septic shock) |
13 (23.6) |
|
Neurologic condition |
7 (12.7) |
|
Othersb
|
6 (10.9) |
|
Reason for tracheostomy |
|
|
Need for long-term free airway maintenance |
11 (20.0) |
|
Prolonged mechanical ventilation |
44 (80.0) |

The average duration of the procedure was 13.3 minutes. With respect to the tube size, 7.5 mm was the most used (60%), and 8.0 mm was the next most used (34.5%). The proportion of tracheostomy oozing was 21.8%, and no major bleeding or pneumothorax occurred. Paratracheal placement of the tracheostomy tube occurred in a patient with a short neck with a high BMI of 33.3 kg/m
2 (
Table 2).
Table 2.
|
Variable |
Value (n=55) |
|
Duration of procedure (min) |
13.3 (4–30) |
|
Tube size (mm) |
|
|
7.0 |
3 (5.5) |
|
7.5 |
33 (60.0) |
|
8.0 |
19 (34.5) |
|
Immediate complication |
|
|
Oozing at the tracheostomy site |
12 (21.8) |
|
Major bleeding |
0 |
|
Paratracheal placement of tracheostomy tube |
1 (1.8) |
|
Pneumothorax |
0 |

Go to :

DISCUSSION
Since PDT is commonly performed at the bedside, the risks and difficulties associated with transporting critically ill patients to the operating room can be avoided [
8]. Several studies have suggested that the incidence of delayed complications, such as tracheal stenosis, is similar between ST and PDT [
9-
11]. Due to these characteristics and ease of the procedure, PDT has become the dominant method of tracheostomy in many centers [
8,
12,
13]. However, conventional PDT has a problem as it requires a lot of equipment and manpower. Especially in infectious diseases, we need to consider how we can solve the problem. In coronavirus disease 2019 (COVID-19) patients, it is recommended that tracheostomy should be performed with the least number of personnel and the most experienced operator should perform the procedure to reduce the number of expose personnel and exposure time [
14,
15].
This study was conducted to confirm the safety and feasibility of the procedure by analyzing all hybrid tracheostomy cases performed in the ICU for 1 year. In this study, hybrid tracheostomy showed several advantages. First, it did not require a lot of manpower and equipment. Second, the procedure could be executed in a short time of about 13 minutes. Considering the above advantages, we think that the hybrid tracheostomy can be a good method for tracheostomy in infectious diseases. This is because compared to the surgical method, the operation time is short, and compared to the conventional PDT, less manpower is required. It means that the exposure time is short, and the number of people exposed is small. Third, no major bleeding events occurred, although approximately one-third of the patients were using antiplatelets or anticoagulants. The proportion of oozing at the tracheostomy site was slightly higher (21.8%), but we presume that this result was obtained because we included all cases in which blood oozing was adequate enough to change the gauze after the procedure based on the records. However, none of the patients required additional hemostatic procedures. However, since there was one case in which the tracheostomy tube was positioned incorrectly in a patient with a short neck and high BMI, it may be difficult to perform this procedure in the following cases: patients with short neck, neck burns, or those who have received neck radiation therapy.
This study has some limitations. First, we were not able to evaluate the long-term complications of this procedure. Second, direct comparison with conventional PDT or ST was not possible because there was no control group. Further, the sampling area was small. Therefore, further research is needed. The final limitation was that surgeons may be more familiar with hybrid tracheostomy than medical intensivist. However, it is not a complicated procedure because the operator only needs to dissect minimal pretracheal tissue to check the trachea. Hence, we think that this method can be performed by any intensivist who can perform conventional PDT methods.
In conclusion, hybrid tracheostomy can be safely performed without any serious complications in most patients. It can also be considered to be a possible treatment for patients taking antiplatelets or anticoagulants without any major complications. It can be a good method in infectious disease because it can reduce the number of exposure personnel and exposure time. However, anatomic considerations, which include but are not limited to short neck, are necessary as patients with a short neck or anatomically difficult structure should undergo ST or PDT with bronchoscopy.
Go to :

HIGHLIGHTS
▪ Hybrid tracheostomy is a method that combines the advantages of percutaneous dilatational tracheostomy and surgical tracheostomy, and it does not need a lot of equipment and experts at the site.
▪ Hybrid tracheostomy can be safely performed without any serious complications in most patients, and it is thought to be a possible treatment for patients taking antiplatelets or anticoagulants.
Go to :






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download