Introduction
Materials and Methods
1. Study group
2. Treatment
Table 1.
| Drug | Dose | Days of administration |
|---|---|---|
| Induction protocol IA | ||
| Prednisone (PO) | 60 mg/m2 per day | 1-28, then taper over 3×3 days |
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | 8, 15, 22, 29 |
| Pirarubicin (IV) | 30 mg/m2 | 8, 15, 22, 29 |
| Pegaspargase (IM) | 2,500 IU/m2 | 1,529 |
| IT chemotherapya) | 1,12,33 | |
| Induction protocol IB | ||
| Cyclophosphamide (IV) | 1,000 mg/m2 per dose | 3,664 |
| Cytarabine (IV) | 75 mg/m2 | 38-41, 45-48, 52-55, 59-62 |
| 6-Mercaptopurine (PO) | 60 mg/m2 | 36-63 |
| IT chemotherapya) | 4,559 | |
| Protocol M | ||
| 6-Mercaptopurine (oral) | 25 mg/m2 per day | 1-56 |
| Methotrexateb) | 5 g/m2 | 8, 22, 36, 50 |
| Methotrexate (IT) | 8, 22, 36, 50 | |
| Reinduction protocol IIA | ||
| Dexamethasone (PO) | 60 mg/m2 per day | 1-21, then taper over 3×3 days |
| Vincristine (IV) | 1.5 mg/m2 (max 2 mg) | 8, 15, 22, 29 |
| Pirarubicin (IV) | 30 mg/m2 | 8, 15, 22, 29 |
| Pegaspargase (IM) | 2,500 IU/m2 | 8 |
| Reinduction protocol IIB | ||
| Cyclophosphamide (IV) | 1,000 mg/m2 per dose | 36 |
| Cytarabine (IV) | 75 mg/m2 per dose | 38-41, 45-48 |
| 6-Thioguanine (PO) | 60 mg/m2 per day | 36-49 |
| IT chemotherapya) | 45, 59 | |
| Maintenance therapy | ||
| Methotrexate (PO) | 20 mg/m2 per dose | Once a week |
| 6-Mercaptopurine (PO) | 50 mg/m2 per day | Daily |
| Methotrexateb) | 5 g/m2 | 4 doses, 3-mo intervals |
3. Response assessment
4. Statistical analysis
5. Ethical statement
Results
1. Clinical manifestation
Table 2.
Values are presented as number (%). NHL-BFM-95, non-Hodgkin lymphoma Berlin–Frankfurt–Münster-95; HyperCVAD/MA, hyperfractionated cyclophosphamide, vincristine, adriamycin, dexamethasone/methotrexate, and cytarabine; ECOG, Eastern Cooperative Oncology Group; T-LBL, T-lymphoblastic lymphoma; B-LBL, B lymphoblastic lymphoma; LDH, lactate dehydrogenase; CNS, central nervous system; IPI, international prognostic index.
2. Treatment outcomes and survival
Fig. 1.
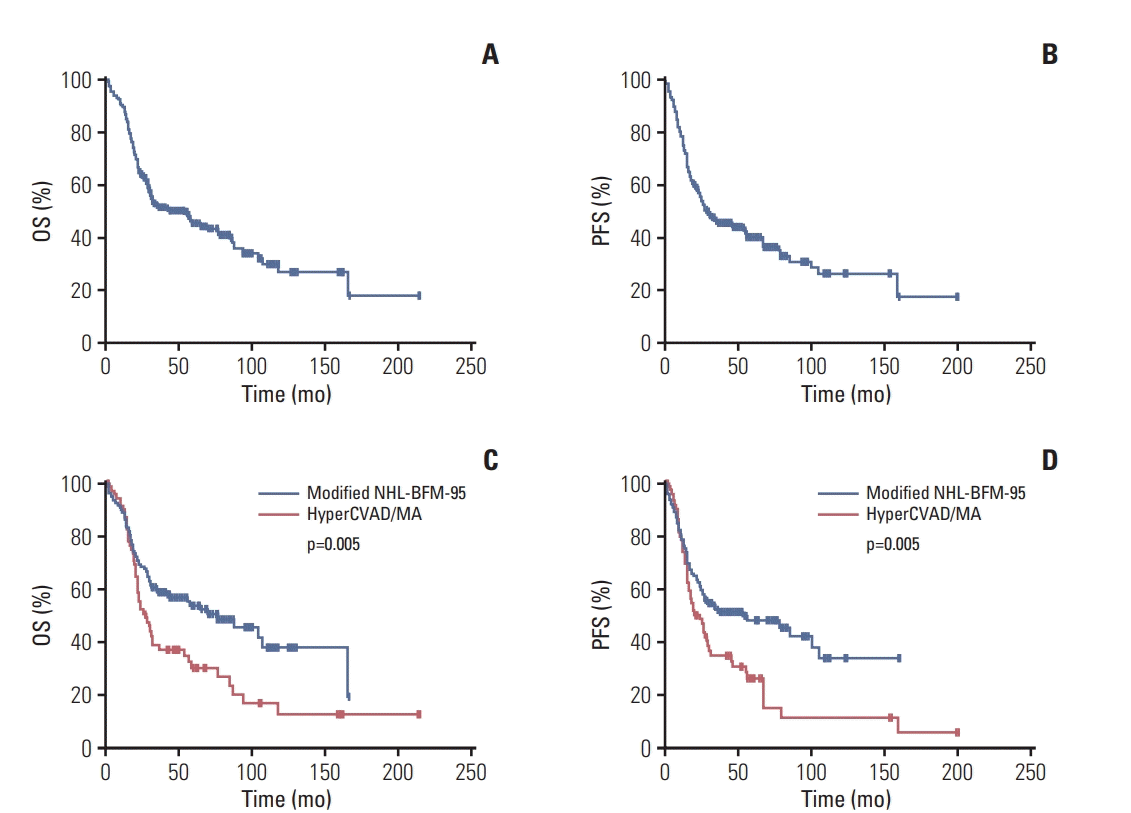
Table 3.
Values are presented as number (%). NHL-BFM-95, non-Hodgkin lymphoma Berlin–Frankfurt–Münster-95; Hyper-CVAD/MA, hyperfractionated cyclophosphamide, vincristine, adriamycin, dexamethasone/methotrexate, and cytarabine; CR, complete remission; MRD, minimal residual disease; OS, overall survival; PFS, progression-free survival.
Table 4.
T-LBL, T-lymphoblastic lymphoma; B-LBL, B lymphoblastic lymphoma; CR, complete remission; MRD, minimal residual disease; NHL-BFM-95, non-Hodgkin lymphoma Berlin–Frankfurt–Münster-95; HyperCVAD/MA, hyperfractionated cyclophosphamide, vincristine, adriamycin, dexamethasone/methotrexate, and cytarabine.
Fig. 2.
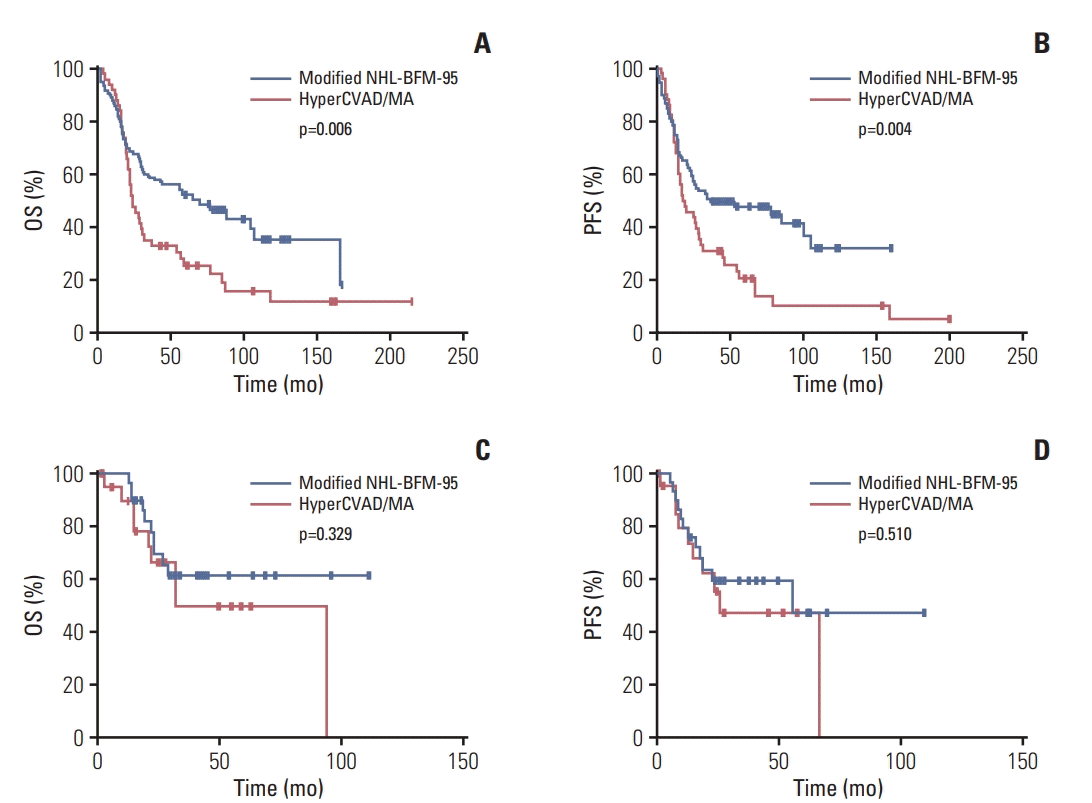
Fig. 3.
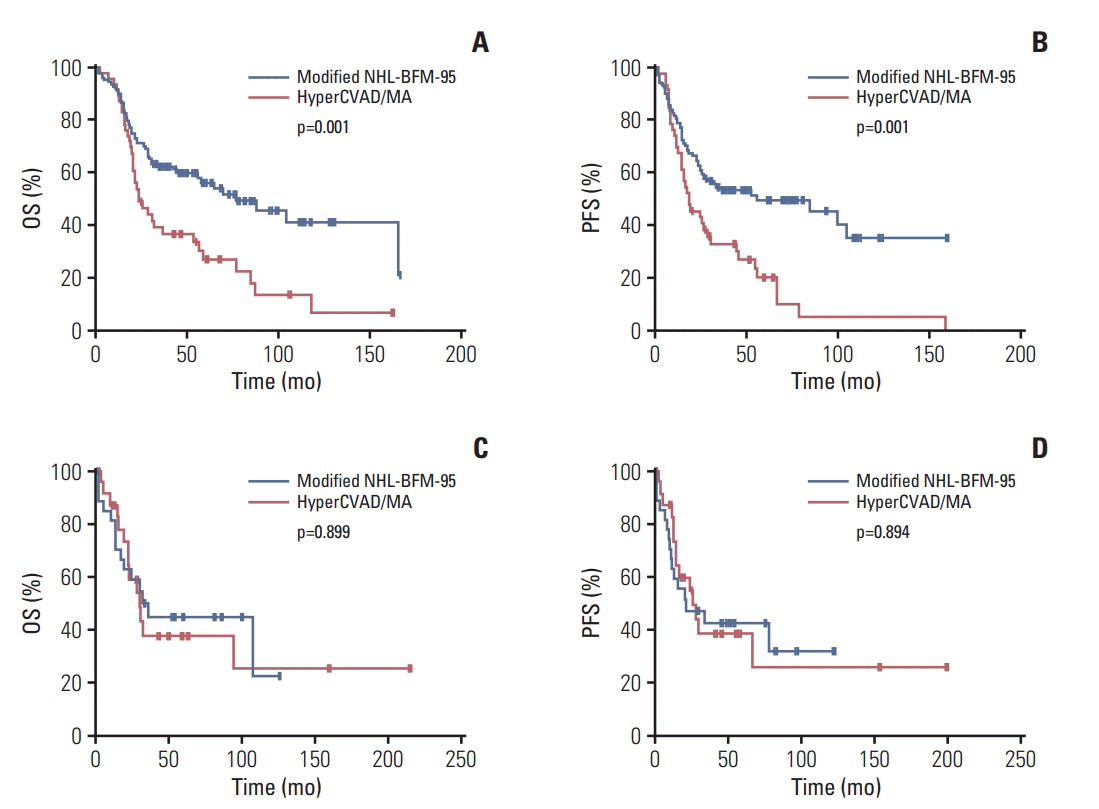
3. Univariate and multivariate analyses
Table 5.
OS, overall survival; PFS, progression-free survival; T-LBL, T-lymphoblastic lymphoma; HR, hazard ratio; CI, confidence interval; NHL-BFM-95, non-Hodgkin lymphoma Berlin–Frankfurt–Münster-95; HyperCVAD/MA, hyper fractionated cyclophosphamide, vincristine, adriamycin, dexamethasone/methotrexate, and cytarabine; ECOG, Eastern Cooperative Oncology Group; WBC, white blood cell; LDH, lactate dehydrogenase; IPI, international prognostic index.
Table 6.
4. Toxicity
Discussion
Table 7.
| Parameter | Modified NHL-BFM-95 | HyperCVAD/MA | CALGB 10403 |
|---|---|---|---|
| No. | 136 | 71 | 295 |
| Median age (yr) | 28 | 32 | 24 |
| Age (%) | |||
| < 40 | 80.1 | 64.8 | 100 |
| ≥ 40 | 19.9 | 35.2 | 0 |
| ECOG | |||
| ≤ 2 | 77.9 | 80.3 | 100 |
| > 2 | 22.1 | 19.7 | 0 |
| Organ dysfunction allowed | No | No | No |
| Ph-positive LBL (%) | 0 | 0 | 31.3 |
| T-cell LBL (%) | 78.7 | 70.4 | 24.1 |
| CR after induction (%) | 77.9 | 66.2 | 89.0 |
| MRD negative after consolidation (%) | 69.1 | 60.1 | 44.0a) |
| 3-Year OS (%) | 58.9 | 38.8 | 73.0 |
| 3-Year PFS (%) | 52.0 | 34.7 | EFS 59.0 |
NHL-BFM-95, non-Hodgkin lymphoma Berlin–Frankfurt–Münster-95; HyperCVAD/MA, hyperfractionated cyclophosphamide, vincristine, adriamycin, dexamethasone/methotrexate, and cytarabine; ECOG, Eastern Cooperative Oncology Group; LBL, lymphoblastic lymphoma; CR, complete remission; MRD, minimal residual disease; OS, overall survival; PFS, progression-free survival.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download