Abstract
Purpose
The purpose of this study was to evaluate clinical characteristics and treatment pattern of ovarian clear cell carcinoma (OCCC) in Korea and the role of adjuvant chemotherapy in early-stage.
Materials and Methods
Medical records of 308 cases of from 21 institutions were reviewed and data including age, performance status, endometriosis, thromboembolism, stage, cancer antigen 125, treatment, recurrence, and death were collected.
Results
Regarding stage of OCCC, it was stage I in 194 (63.6%), stage II in 34 (11.1%), stage III in 66 (21.6%), and stage IV in 11 (3.6%) patients. All patients underwent surgery. Optimal surgery (residual disease ≤ 1 cm) was achieved in 89.3%. Majority of patients (80.5%) received postoperative chemotherapy. The most common regimen was taxane-platinum combination (96%). Median relapse-free survival (RFS) was 138.5 months for stage I, 33.4 for stage II, 19.3 for stage III, and 9.7 for stage IV. Median overall survival (OS) were not reached, 112.4, 48.7, and 18.3 months for stage I, II, III, and IV, respectively. Early-stage (stage I), endometriosis, and optimal debulking were identified as favorable prognostic factors for RFS. Early-stage and optimal debulking were also favorable prognostic factors for OS. Majority of patients with early-stage received adjuvant chemotherapy. However, additional survival benefit was not found in terms of recurrence.
Conclusion
Majority of patients had early-stage and received postoperative chemotherapy regardless of stage. Early-stage and optimal debulking were identified as favorable prognostic factors. In stage IA or IB, adding adjuvant chemotherapy did not show difference in survival. Further study focusing on OCCC is required.
Globally ovarian cancer is the 7th leading cause of cancer-related death among women. In Korea, it is the 10th common female cancer. Its incidence is continuously increasing by 1.6% of annual percentage change [1]. Epithelial ovarian cancer is a heterogeneous group with eight histologic subtypes according to World Health Organization classification. Although these subtypes have different biology, they have been treated in the same way since clinical trials have mostly included serous carcinoma, the most common histologic subtype.
Ovarian clear cell carcinoma (OCCC) accounts for 3%-10% of epithelial carcinoma. Significant geographic difference has been noted in the prevalence of OCCC [2]. The prevalence is higher in Japanese and Asian populations than in Western countries [2]. A recent Japanese study has reported that OCCC is increased significantly, accounting for up to 30% of epithelial ovarian cancer [3]. Several social-environmental factors, related to ovulation and menstruation, have been suggested as the reasons for the increasing incidence of OCCC [3]. According to Korean Central Cancer Registry, the proportion of OCCC was 11.6% [1]. Compared to high-grade serous carcinoma (HGSC), OCCC usually presents at younger age and lower stage. OCCC is known to be associated with endometriosis and putative precursor lesion [2,4]. It has a high frequency of thromboembolic complication [2,4]. Early-stage OCCC confined to ovary has favorable prognosis. However, OCCC in advanced stage has poor prognosis due to its inherent chemoresistance. Notwithstanding its chemoresistance and good prognosis in early stage, adjuvant chemotherapy in early-stage OCCC is commonly used and conflicting data have been reported [5,6]. In terms of genetic profile, PIK3CA and ARID1A mutations at high frequency have been noted, while BRCA mutation and TP53 mutation at low frequency are commonly found in HGSC [7-11]. Hence, treatment for OCCC that is different from HGSC is needed.
Thus, the objective of this study was to evaluate clinical characteristics and treatment pattern of OCCC in Korea. Additionally, the role of adjuvant chemotherapy in early-stage OCCC was assessed.
This was a retrospective study of 308 cases of clear cell ovarian carcinoma from 21 institutions in South Korea between January 1995 and December 2015. All patients underwent surgery and had histologically confirmed pure clear cell ovarian carcinoma. Medical records were reviewed. Data including age, Eastern Cooperative Oncology Group (ECOG) performance status, presence of endometriosis and history of thromboembolism (TE), stage of OCCC, initial level of cancer antigen 125 (CA-125), treatment (surgery, chemotherapy), recurrence, and death were collected. Presence of endometriosis was checked according to pathologic report from surgery. Surgical staging was done according to International Federation of Gynecology and Obstetrics (FIGO) guidelines for ovarian cancer (8th edition, 2017). Optimal surgery was defined as residual disease ≤ 1 cm.
Relapse-free survival (RFS) and overall survival (OS) were determined from the date of pathologic diagnosis to the date of recurrence or death using the Kaplan-Meier method. Survival rate was derived from life table. To evaluate prognostic factors for RFS and OS, univariate and multivariate Cox regression analyses were done. Univariate analyses were performed with factors including age, performance status, stage, histologic grade, endometriosis, TE, optimal debulking and postoperative chemotherapy. Multivariate analyses were done with factors of p-value of < 0.1 at univariate analyses. All statistical analyses were performed using Statistical Package for Social Sciences (SPSS/PC+ 18.0, Chicago, IL), and a p-value of less than 0.05 was considered statistically significant.
Three hundred and eight patients were included in this study. Baseline characteristics of these patients are summarized in Table 1. Their median age at diagnosis was 51 years (range, 25 to 81 years). The majority of patients (78.6%) had ECOG performance status of grade 0 or 1. Regarding the stage of disease (n=303), it was stage I in 194 (63.7%), stage II in 34 (11.1%), stage III in 66 (21.6%), and stage IV in 11 (3.6%). Median CA-125 level was 72.3 IU/mL (range, 1.9 to 8,930 IU/mL) in all patients. It was 45.7 IU/mL in stage I, 98.9 IU/mL in stage II, 192.1 IU/mL in stage III, and 694.8 IU/mL in stage IV. About one-third of patients (34.9%) had co-existing endometriosis and 19 patients (6.2%) had history of TE. Histologic tumor grading was done for 141 patients and grade 3 in 81 patients (45.9%).
Table 2 shows treatment pattern for OCCC. Eight patients (2.6%) received neoadjuvant chemotherapy (Table 2). All patients underwent surgery. Two-hundred and seventy-seven patients (89.9%) underwent total hysterectomy including previous hysterectomy, both salpingo-oophrectomy, omentectomy, and pelvic lymph node dissection. The others underwent unilateral salpingo-oophorectomy with or without hysterectomy. They were all young aged (under 40) and had stage I disease. Optimal surgery was achieved in 275 patients (89.3%). Postoperative chemotherapy was administered in 248 patients (80.5%). The most commonly used regimen was taxane-platinum combination (96%). The median number for administered cycles of chemotherapy was 6 (range, 1 to 12).
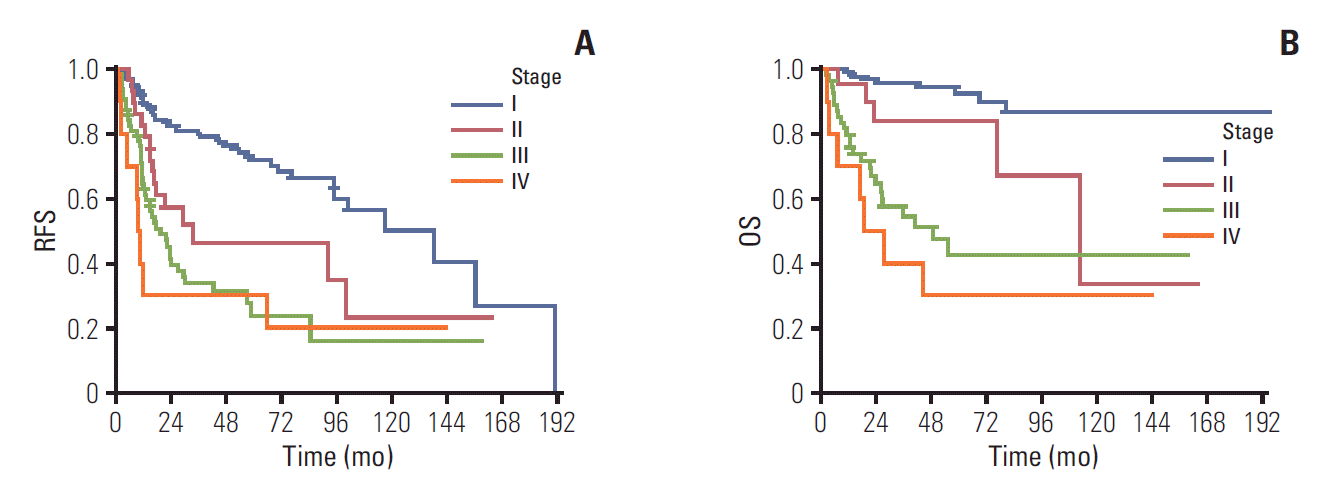
Median follow-up duration was 31.2 months (range, 0.5 to 195.4 months). Recurrence occurred in 119 patients (40.2%). Twelve cases (3.9%) had missing information for recurrence or progression and 72 cases (23.4%) had missing information for survival. Median RFS for stage I, II, III, and IV were 138.5 months (95% confidence interval [CI], 87.8 to 189.2), 33.4 months (95% CI, 0 to 97.1), 19.3 months (95% CI, 4.5 to 10.5), and 9.7 months (95% CI, 7.9 to 11.4), respectively (log-rank p < 0.001) (Fig. 1A). Median OS was not reached in stage I, 112.4 months (95% CI, 59.5 to 165.3) in stage II, 48.7 months (95% CI, 18.8 to 78.7) in stage III, and 18.3 months (95% CI, 2.5 to 34.1) in stage IV (log-rank p < 0.001) (Fig. 1B). One-year RFS or progression-free survival rates for stage I, II, III, and IV were 90%, 83%, 63%, and 30% in stage I, II, III, and IV, respectively. Three-year RFS rates for stage I, II, III, and IV were 80%, 47%, 34%, and 30%, respectively. OS rates at 1-year was 99%, 95%, 80%, and 70%, res-pectively. These rates at 3-year were 96%, 85%, 54%, and 40% for stage I, II, III, and IV, respectively.
In univariate analyses, early-stage (I), endometriosis, optimal debulking (residual disease ≤ 1 cm), and adding postoperative chemotherapy were favorable prognostic factors for RFS. Early-stage, optimal debulking, and adding postoperative chemotherapy were also significant prognostic factors for OS. In multivariate analyses, early-stage, endometriosis, and optimal debulking remained as favorable prognostic factors for RFS. Early-stage, and optimal debulking predicted longer OS (Table 3).
Role of adjuvant chemotherapy in patients with early-stage OCCC was evaluated. Ninety-four patients (30.5%) had stage IA or IB disease, and 77 patients (81.9%) received adjuvant chemotherapy. Adjuvant chemotherapy was administered in 69 patients (81.2%) with stage IA (n=95), and eight (88.9%) with stage IB (n=9). Median RFS was 95.2 months in patients with adjuvant chemotherapy. It was not reached in patients without adjuvant chemotherapy (p=0.57). Median OS was not reached.
The aim of the present study was to assess clinical features and prognosis of Korean OCCC and study the role of adjuvant chemotherapy in early-stage OCCC. Similar to global epidemiology of OCCC, majority of Korean OCCC patients presented at younger age (median, 51 years) and early stage. About three-quarter of patients had stage I or II disease. According to the Surveillance, Epidemiology, and End Results (SEER) data, the incidence of OCCC in epithelial ovarian cancer was different according to ethnicity, 4.8% in whites, 3.1% in blacks, and 11.1% in Asians [12]. Machida et al. [3] have reported recent trends of epithelial ovarian cancer in Japan. They found the significant increase of OCCC in recent years, and an incidence of about 30% for epithelial ovarian cancer. Moreover, patients aged between 30 and 50 showed similar incidence of OCCC with serous carcinoma [3]. Several factors are responsible for the increase of OCCC, including earlier menarche, lower use of oral contraceptives (OC) compared to western countries, and low pregnancy rate. Those could increase the number of ovulations in lifetime which in turn raise the risk of endometriosis, the known precursor of OCCC. Compared to Caucasians or African Americans, Asian women seem to have higher prevalence of endometriosis, although medical utilization may account partly for the difference [13]. In Korea, OCCC accounts for 11.6% of epithelial ovarian cancer, not as high as that in Japan. However, its incidence has been increased continuously at an annual percentage change of 1.6%. According to Kim et al. [14], the incidence of OCCC in Korea has increased significantly since 1999. Current Korean trends and status in terms of pregnancy, menarche, and the use of OC are similar to those in Japan. Thus continuous increase of OCCC in Korea is expected.
The association of endometriosis and OCCC has been studied widely. Endometriosis is accepted as a precursor lesion of OCCC. Son et al. [15] recommended active surveillance with at least 1-year interval in asymptomatic patients with endometriosis. In terms of prognosis, conflicting data have been reported. OCCC with endometriosis has been reported to be associated with early stage and good prognosis [16,17]. Meanwhile no difference in prognosis of OCCC according to the presence of endometriosis has been reported [18,19]. In the present study, about one-third of patients had endometriosis. These patients showed longer median RFS (median, not reached vs. 67.5 months; log-rank p=0.029) and OS (not reached; log-rank p=0.054), although the difference in OS was not statistically significant. OCCC patients are known to be at high risk of venous TE (15%-42%). Negative impact of TE on prognosis has been reported [20,21]. The incidence of venous TE has been reported to be more than two times more compared to that of serous carcinoma, with those with advanced stage having higher risk [20]. Thus in recurrent OCCC patients, life-long anticoagulation is recommended [20]. In the current study 6.2% patients had TE. No prognostic role of TE was revealed. Due to the retrospective nature of this study, its incidence might have been underestimated.
Oliver et al. [22] reported that OCCC in early stage has better prognosis than serous carcinoma. However, it has significant poorer prognosis in advanced stage. Progression-free survival rate and survival rate in early-stage (I, II) OCCC at 5-year were 75% and 80%, respectively, compared to 63% and 78% in serous carcinoma [22]. In the present study, relapse-free survival rate and OS rate at 5-year for stage I and II were 68% and 91%, respectively, showing good prognosis of early-stage OCCC.
International Collaborative Ovarian Neoplasm Trial 1 (ICON 1) has evaluated adjuvant chemotherapy in earlystage epithelial cancer [23]. Ten-year follow-up results confirmed the benefit of adjuvant chemotherapy [23]. OCCC is classified as high risk of recurrence. Thus adjuvant chemotherapy is recommended regardless of stage or surgical result (optimal or suboptimal) [23,24]. In ICON 1 trial, OCCC accounted for 12% of enrolled patients, and more than 80% of patients had serous, mucinous or endometrioid histology [25]. Using SEER data, Oseledchyk et al. [6] have assessed adjuvant chemotherapy in stage I OCCC. Their study included 1,995 patients with stage I OCCC and found that adjuvant chemotherapy was not associated with improved OS. In the present study, over 80% of patients received postoperative chemotherapy. The proportion was similar for all stages. In stage IA or IB patients, adjuvant chemotherapy was not related to longer RFS. Considering the fact that minority of patients with OCCC were included in ICON 1 trial and that OCCC has distinct biology including intrinsic chemoresistance, genetic profile, and good prognosis in early stage, the role of adjuvant chemotherapy in early-stage OCCC should be reconsidered.
Due to the nature of this retrospective study from multicenter, there were missing data. In addition, the completeness of optimal surgical staging including inspection and palpation of all peritoneal surfaces; biopsies of any suspect lesions for metastases; peritoneal washing; infracolic omentectomy; (blind) biopsies of right hemidiaphragm, of right and left paracolic gutter, of pelvic sidewalls, of ovarian fossa, of bladder peritoneum, and of cul-de-sac; sampling of iliac and periaortic lymph nodes is unclear.
In conclusion, this study showed clinical features, treatment patterns and prognosis of OCCC in Korea. Majority of patients had early stage and received postoperative chemotherapy regardless of stage. Early stage (stage I), and optimal debulking (residual disease < 1 cm) were identified as favorable prognostic factors for RFS and OS. Patients with endometriosis showed better prognosis. In patients with stage IA and IB, adding adjuvant chemotherapy did not show difference in survival. Considering the distinct biology of OCCC and its continuous increasing incidence, particularly in Asian, further study focusing on OCCC is required.
References
1. Korea Central Cancer Registry, National Cancer Center. Annual report of cancer statistics in Korea in 2015. Sejong: Ministry of Health and Welfare;2017.
2. Okamoto A, Glasspool RM, Mabuchi S, Matsumura N, Nomura H, Itamochi H, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for clear cell carcinoma of the ovary. Int J Gynecol Cancer. 2014; 24(9 Suppl 3):S20–5.

3. Machida H, Matsuo K, Yamagami W, Ebina Y, Kobayashi Y, Tabata T, et al. Trends and characteristics of epithelial ovarian cancer in Japan between 2002 and 2015: a JSGO-JSOG joint study. Gynecol Oncol. 2019; 153:589–96.

4. Gadducci A, Lanfredini N, Tana R. Novel insights on the malignant transformation of endometriosis into ovarian carcinoma. Gynecol Endocrinol. 2014; 30:612–7.

5. Hogen L, Brar H, Covens A, Bassiouny D, Bernardini MQ, Gien LT, et al. Is adjuvant chemotherapy beneficial for surgical stage I ovarian clear cell carcinoma? Gynecol Oncol. 2017; 147:54–60.

6. Oseledchyk A, Leitao MM Jr, Konner J, O'Cearbhaill RE, Zamarin D, Sonoda Y, et al. Adjuvant chemotherapy in patients with stage I endometrioid or clear cell ovarian cancer in the platinum era: a Surveillance, Epidemiology, and End Results Cohort Study, 2000-2013. Ann Oncol. 2017; 28:2985–93.

7. Jones S, Wang TL, Shih IM, Mao TL, Nakayama K, Roden R, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010; 330:228–31.
8. Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010; 363:1532–43.
9. Ayhan A, Mao TL, Seckin T, Wu CH, Guan B, Ogawa H, et al. Loss of ARID1A expression is an early molecular event in tumor progression from ovarian endometriotic cyst to clear cell and endometrioid carcinoma. Int J Gynecol Cancer. 2012; 22:1310–5.

10. Katagiri A, Nakayama K, Rahman MT, Rahman M, Katagiri H, Nakayama N, et al. Loss of ARID1A expression is related to shorter progression-free survival and chemoresistance in ovarian clear cell carcinoma. Mod Pathol. 2012; 25:282–8.

11. Yamamoto S, Tsuda H, Takano M, Tamai S, Matsubara O. Loss of ARID1A protein expression occurs as an early event in ovarian clear-cell carcinoma development and frequently coexists with PIK3CA mutations. Mod Pathol. 2012; 25:615–24.

12. Anglesio MS, Carey MS, Kobel M, Mackay H, Huntsman DG; Vancouver Ovarian Clear Cell Symposium Speakers. Clear cell carcinoma of the ovary: a report from the first Ovarian Clear Cell Symposium, June 24th, 2010. Gynecol Oncol. 2011; 121:407–15.

13. Yamamoto A, Johnstone EB, Bloom MS, Huddleston HG, Fujimoto VY. A higher prevalence of endometriosis among Asian women does not contribute to poorer IVF outcomes. J Assist Reprod Genet. 2017; 34:765–74.

14. Kim SI, Lim MC, Lim J, Won YJ, Seo SS, Kang S, et al. Incidence of epithelial ovarian cancer according to histologic subtypes in Korea, 1999 to 2012. J Gynecol Oncol. 2016; 27:e5.

15. Son JH, Yoon S, Kim S, Kong TW, Paek J, Chang SJ, et al. Clinicopathologic characteristics of ovarian clear cell carcinoma in the background of endometrioma: a surveillance strategy for an early detection of malignant transformation in patients with asymptomatic endometrioma. Obstet Gynecol Sci. 2019; 62:27–34.

16. Bai H, Cao D, Yuan F, Sha G, Yang J, Chen J, et al. Prognostic value of endometriosis in patients with stage I ovarian clear cell carcinoma: experiences at three academic institutions. Gynecol Oncol. 2016; 143:526–31.

17. Kim HS, Kim MA, Lee M, Suh DH, Kim K, No JH, et al. Effect of endometriosis on the prognosis of ovarian clear cell carcinoma: a two-center cohort study and meta-analysis. Ann Surg Oncol. 2015; 22:2738–45.

18. Orezzoli JP, Russell AH, Oliva E, Del Carmen MG, Eichhorn J, Fuller AF. Prognostic implication of endometriosis in clear cell carcinoma of the ovary. Gynecol Oncol. 2008; 110:336–44.

19. Park JY, Kim DY, Suh DS, Kim JH, Kim YM, Kim YT, et al. Significance of ovarian endometriosis on the prognosis of ovarian clear cell carcinoma. Int J Gynecol Cancer. 2018; 28:11–8.

20. Duska LR, Garrett L, Henretta M, Ferriss JS, Lee L, Horowitz N. When 'never-events' occur despite adherence to clinical guidelines: the case of venous thromboembolism in clear cell cancer of the ovary compared with other epithelial histologic subtypes. Gynecol Oncol. 2010; 116:374–7.

21. Ye S, Yang J, Cao D, Bai H, Huang H, Wu M, et al. Characteristic and prognostic implication of venous thromboembolism in ovarian clear cell carcinoma: a 12-year retrospective study. PLoS One. 2015; 10:e0121818.

22. Oliver KE, Brady WE, Birrer M, Gershenson DM, Fleming G, Copeland LJ, et al. An evaluation of progression free survival and overall survival of ovarian cancer patients with clear cell carcinoma versus serous carcinoma treated with platinum therapy: an NRG Oncology/Gynecologic Oncology Group experience. Gynecol Oncol. 2017; 147:243–9.

23. Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013; 24 Suppl 6:vi24–32.

24. National Comprehensive Cancer Network. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer (version 1.2019) [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network;2019. [cited 2019 Apr 18]. Available from: https://www.nccn.org/.
25. Trimbos JB, Parmar M, Vergote I, Guthrie D, Bolis G, Colombo N, et al. International Collaborative Ovarian Neoplasm trial 1 and Adjuvant ChemoTherapy In Ovarian Neoplasm trial: two parallel randomized phase III trials of adjuvant chemotherapy in patients with early-stage ovarian carcinoma. J Natl Cancer Inst. 2003; 95:105–12.
Table 1.
Patient characteristics
Table 2.
Summary of treatments
Table 3.
Prognostic factors for RFS




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download