Abstract
Background
Elizabethkingia meningoseptica, formerly known as Chryseobacterium meningosepticum, is a non-motile, non-fastidious, catalase and oxidase-positive, aerobic, glucose-non-fermentative, Gram-negative bacillus that was first defined by Elizabeth O. King in 1959. It has emerged as an opportunistic pathogen that has infected patients in extreme age groups and immunocompromised individuals, especially in intensive care settings. There has been an increased interest in this pathogen due to its increasing occurrence around the world, ubiquitous nature, and inherent capacity for antimicrobial resistance.
Methods
We describe an observational study at a tertiary care center in Karachi, Pakistan, based on patients admitted between January 2013 and December 2018, with E. meningoseptica infections. All patients were confirmed to have a positive clinical culture specimen for E. meningoseptica along with symptoms and signs consistent with infection. Data were collected on a structured proforma from the Hospital Information Management Systems.
Results
Sixteen patients with E. meningoseptica that met the criteria for infection were identified, 13 of whom required admission. Eight patients had bacteremia in addition to confirmed E. meningoseptica infection. Two of the isolates were multi-drug resistant and only sensitive to minocycline. Nine out of 13 patients that were admitted required intubation and mechanical ventilation. The median length of hospital stay was 13 days, and five out of the 13 patients died during the hospital stay.
Elizabethkingia meningoseptica, formerly known as Chryseobacterium meningosepticum, is a non-motile, non-fastidious, catalase and oxidase-positive, aerobic, glucose-non-fermentative, Gram-negative bacillus that was first defined by Elizabeth O. King in 1959 [1]. The Elizabethkingia genus has been noted due to the genetic makeup that facilitates a large degree of genetic variability and subsequent antimicrobial resistance. This, combined with lack of literature reports on the wide distribution in nature and of adequate treatment regimens have led to high mortality rates in hospital-settings, particularly in intensive care units (ICUs), since 2004 [2].
E. meningoseptica is isolated most frequently from soil, saltwater, and freshwater and from dry and moist clinical environmental and equipment surfaces, intravenous lipid solutions, and municipal water supplies, including those adequately chlorinated [3]. Although nearly ubiquitous in nature, it is an uncommon human pathogen. E. meningoseptica predominately causes outbreaks of meningitis in immunocompromised patients, particularly in premature newborns and infants in neonatal ICUs of developing countries [4]. The bacterium is a rare cause of nosocomial pneumonia, endocarditis, and meningitis in immunocompromised adults. More recently, in the past few years, it has been found to cause soft tissue infection and sepsis in immunocompetent adults [5].
In a study from Wisconsin, 48 cases of Elizabethkingia infection were reported during an outbreak, which resulted in 17 deaths in a 5-month period beginning in November 2015 [6]. Another case series showed that the yearly incidence of E. meningoseptica bacteremia increased substantially from 2002 to 2006 (from 6.8–13.1 to 26.6–39.9 per 100,000 admissions; P = 0.006) [7].
Moreover, Elizabethkingia, similar to its genetic relative Chryseobacterium species, is inherently extensively drug resistant. It is resistant to a broad spectrum of antibiotic classes, including macrolides, tetracyclines, linezolid, polymyxin group, chloramphenicol, aminoglycosides, and beta-lactam drugs [8]. Vancomycin, rifampicin, new fluoroquinolones, piperacillin-tazobactam, and minocycline are the current preferred empirical choices for treating E. meningoseptica infections.
E. meningoseptica is an important emerging opportunistic bacterium that primarily occurs in nosocomial settings. It is unclear which treatment regimen is most effective and what factors are associated with adverse outcomes. The aim of this study is to describe the clinical features and outcomes of E. meningoseptica infections in a tertiary care center in Karachi, Pakistan.
The study received an exemption from ethical approval from the Aga Khan University Ethics Review Committee (ERC #2019-1786-4439) and requirement of informed consent was waived due to retrospective nature of study. Data was anonymized and no personal identifiers were collected.
This was an observational study of patients admitted between January 2013 and December 2018, with E. meningoseptica infections. All patients were confirmed to have a positive clinical culture specimen for E. meningoseptica along with signs and symptoms consistent with infection. All patients presumed to be colonized but not infected were excluded. Data were collected on a structured proforma from the Hospital Information Management Systems. Identification and susceptibility of E. meningoseptica isolated from cultures were performed by automated systems in accordance with Clinical Laboratory Standards Institute recommendations. Identification of E. meningoseptica was determined by a Vitek 2 system (bioMerieux, Marcy-l’Étoile, France).
Infection was defined based on clinical presentation along with patient-related factors and microbiological diagnosis. The Center for disease control/National Healthcare Safety Network definitions [9] for specific types of infections were used. A multidisciplinary team of doctors including infectious disease consultants, pulmonologists, and intensivists was involved in case identification and management. Patients were considered to be colonized if they had positive culture results for the organism but no signs and symptoms to suggest active infection based on the primary physician evaluation and continued status of good health without treatment. Patients were considered to have co-infection if a clinically significant true pathogen was identified simultaneously from a culture specimen and the patient improved after corresponding treatment. Patients were considered to have co-colonization if the pathogen isolated was a known contaminant or did not produce signs and symptoms consistent with infection. Polymicrobial infection was defined as the presence of another bacteria or fungi in the same culture specimen. Multi-drug resistance was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories [10]. Death was confirmed as all-cause mortality during hospitalization.
Data were analyzed using IBM SPSS ver. 19 (IBM Corp., Armonk, NY, USA). Descriptive analysis was performed for demographic features with median and interquartile range values reported for quantitative variables such as age and length of hospital stay, and frequency (percentage) were reported for qualitative variables such as sex, comorbid conditions, mortality, and complications. A P-value ≤ 0.05 was considered statistically significant. Data were kept confidential, and no personal identifiers were used.
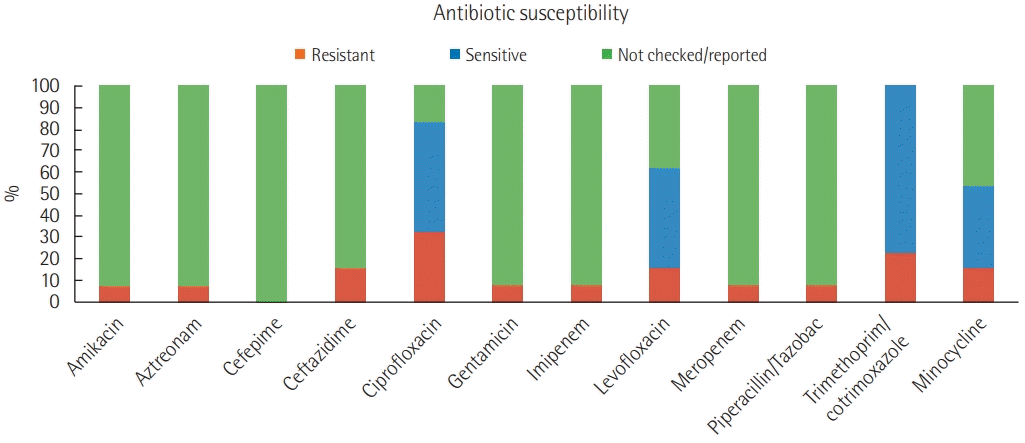
Sixteen patients with E. meningoseptica infections were identified between 2013 and 2018, 13 of whom required hospital admission, constituting an infection rate of 2.9 per 100,000 admissions. The median age was 29 years for six males and seven females, with ages ranging from 3 days to 83 years. The most common comorbid conditions were diabetes (5/13) and hypertension (5/13). The average Charlson’s comorbidity index was 3.3. Three patients had underlying malignancy, and five patients had a history of repeated hospitalization. Eleven patients were admitted to the ICU. Eight of 13 patients had bacteremia with E. meningosepticum, and the most common source of bacteremia was central line-associated bloodstream infection in six patients. Among other sites of infection, one had urinary tract infection, two had meningitis, two had pneumonia, and one had infective endocarditis. Nine patients had mono-microbial growth, whereas four had polymicrobial growth in culture. Of 13 patients, eight required a Foley catheter and nine required a central venous catheter, endotracheal intubation, and mechanical ventilation. The clinical characteristics of all 13 patients are summarized in Table 1. E. meningosepticum was sensitive to quinolones in five of 13 isolates. Two of the E. meningosepticum isolates were multi- drug resistant with sensitivity only to minocycline, which was the drug used for definitive treatment. The susceptibility pattern of all isolates is summarized in Figure 1. Five of 13 patients were treated with a quinolone, four with cotrimoxazole, and four with minocycline. However, culture appropriate antibiotics were not initiated in two patients. The most common co-infection was with multi-drug resistant Acinetobacter (n = 2) or Pseudomonas aeruginosa (n = 2). Five of 13 patients died, two cases were referred for palliative care in view of advanced underlying malignancy, and the median length of hospital stay was 13 days. All five patients who died had a history of repeat hospitalization and significant comorbidities. All five of these patients were mechanically ventilated and underwent invasive catheterization. E. meningoseptica was isolated from the blood of three patients and from tracheal aspirate in one patient who had undergone esophagectomy, thoracotomy, and tracheostomy. Death could be attributed to E. meningoseptica infection in two of five patients. One patient had repeated isolation of the organism from blood cultures due to infective endocarditis, and the other had nosocomial pneumonia after prolonged hospitalization.
Our study identified and analyzed Elizabeth meningoseptica infections in patients with underlying comorbidities, particularly in patients that required frequent hospitalizations. While many isolated case reports and series have been reported [11- 16], only a few [17] have included more than four Elizabethkingia cases (Table 2). There are no reports on Elizabethkingia cases from Pakistan. Our study identified 16 patients in the last six years with Elizabethkingia meningosepticum infections. Unlike other case series with predominantly neonatal cases [17], our patients had a median age of 29 years. Similar to other case reports, the infections were urinary tract infection and meningitis [11,16]. The outcomes have been variable, with significant mortality among neonates [17]. However, patients with isolates susceptible to quinolones and treated with Ciprofloxacin had better survival outcomes [14]. This result is similar to patients that of who were treated with a quinolone in our study.
E. meningosepticum is resistant to most commonly used antimicrobials; therefore, options for treatment are limited. Most studies have established that clinical isolates are resistant to aminoglycosides and beta lactams, with notable exception of piperacillin-tazobactam, and show sensitivities to fluroquinolones and tetracyclines [15]. These patterns were consistent with the results of this study.
This study is a single-center case series with several inherent limitations such as the small sample size and unavailability of molecular testing and minimum inhibitory concentration data on all isolates. However, this study is the largest to date and the first from Pakistan, to report clinical features and treatment outcomes of E. meningoseptica infections. Moreover, it offers insight into the sensitivity pattern of this organism in our region. E. meningosepticum is an emerging nosocomial pathogen that causes mortality in patients requiring intensive care.
▪ Elizabethkingia meningoseptica is an opportunistic pathogen infecting people in the extremes of age and the immunocompromised, especially in intensive care settings.
▪ We are describing the largest case series to date reporting E. meningoseptica infections.
▪ Our study found that Chryseobacterium meningosepticum is an emerging nosocomial pathogen causing mortality in patients requiring intensive care and outcomes are better if isolates are susceptible to quinolones.
REFERENCES
1. Kim KK, Kim MK, Lim JH, Park HY, Lee ST. Transfer of Chryseobacterium meningosepticum and Chryseobacterium miricola to Elizabethkingia gen. nov. as Elizabethkingia meningoseptica comb. nov. and Elizabethkingia miricola comb. nov. Int J Syst Evol Microbiol. 2005; 55(Pt 3):1287–93.

2. Teo J, Tan SY, Liu Y, Tay M, Ding Y, Li Y, et al. Comparative genomic analysis of malaria mosquito vector-associated novel pathogen Elizabethkingia anophelis. Genome Biol Evol. 2014; 6:1158–65.

3. Jean SS, Lee WS, Chen FL, Ou TY, Hsueh PR. Elizabethkingia meningoseptica: an important emerging pathogen causing healthcare-associated infections. J Hosp Infect. 2014; 86:244–9.

4. Mukerji R, Kakarala R, Smith SJ, Kusz HG. Chryseobacterium indologenes: an emerging infection in the USA. BMJ Case Rep. 2016; 2016:bcr2016214486.

5. Tuon FF, Campos L, Duboc de Almeida G, Gryschek RC. Chryseobacterium meningosepticum as a cause of cellulitis and sepsis in an immunocompetent patient. J Med Microbiol. 2007; 56(Pt 8):1116–7.

6. Meyers SL. A crash course In Elizabethkingia, the rare bacterial infection spreading across Wisconsin [Internet]. Madison (WI): Wisconsin Public Radio;2016. [cited 2021 Jul 1]. Available from: https://www.wpr.org/crash-course-elizabethkingia-rare-bacterial-infection-spreading-across-wisconsin.
7. Hsu MS, Liao CH, Huang YT, Liu CY, Yang CJ, Kao KL, et al. Clinical features, antimicrobial susceptibilities, and outcomes of Elizabethkingia meningoseptica (Chryseobacterium meningosepticum) bacteremia at a medical center in Taiwan, 1999-2006. Eur J Clin Microbiol Infect Dis. 2011; 30:1271–8.

8. Loch TP, Faisal M. Emerging flavobacterial infections in fish: a review. J Adv Res. 2015; 6:283–300.

9. National Healthcare Safety Network. CDC/NHSN surveillance definitions for specific types of infections [Internet]. Atlanta (GA): Centers for Disease Control and Prevention;2021. [cited 2021 Jun 5]. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf.
10. Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012; 18:268–81.

11. Khan ID, Lall M, Sen S, Ninawe SM, Chandola P. Multiresistant Elizabethkingia meningoseptica infections in tertiary care. Med J Armed Forces India. 2015; 71:282–6.

12. Arbune M, Fotea S, Nechita A, Stefanescu V. Emerging infection with Elizabethkingia meningoseptica in neonate: a case report. J Crit Care Med (Targu Mures). 2018; 4:96–100.

13. Celik K, Terek D, Olukman O, Gulfidan G, Calkavur S, Devrim I, et al. Colonization and infection with a rare microorganism in a neonatal intensive care unit: three preterm infants with Elizabethkingia meningoseptica. Arch Argent Pediatr. 2019; 117:e631–4.

14. Hamza WS, Morsi SS, Al Roomi ES, Rotimi VO. Epidemiological analysis of Elizabethkingia meningoseptica infection cluster among mechanically ventilated pediatric intensive care patients. Int J Community Med Public Health. 2018; 5:3212–9.

15. Sahu MK, Balasubramaniam U, C B, Singh SP, Talwar S. Elizabethkingia meningoseptica: an emerging nosocomial pathogen causing septicemia in critically ill patients. Indian J Crit Care Med. 2019; 23:104–5.
Table 1.
Summary of patients with Elizabethkingia meningoseptica infections
Table 2.
Summary of studies reporting Elizabethkingia infections and outcomes
| Study | Sample size | Clinical diagnosis | Risk factor | Treatment | Outcome |
|---|---|---|---|---|---|
| Khan et al. [11] (2015) | 4 | Empyema, peritonitis, endocarditis, intra-abdominal sepsis | CKD, valvular heart disease, ruptured ectopic pregnancy | Cotrimoxazole-sensitive isolates | 3 Survived; 1 died |
| Arbune et al. [12] (2018) | 1 | Meningitis | Preterm baby | Piperacillin, tazobactam, and Rifampin | Survived |
| Celik et al. [13] (2019) | 3 | Ventilator-associated pneumonia | Preterm infants | One baby treated with cefoperazone-sulbactam and 2 babies treated with ciprofloxacin | All survived |
| Hamza et al. [14] (2018) | 4 | Central line-associated bloodstream infection and hospital-acquired pneumonia | Pediatric patients requiring intensive care | Treatment details not available | All survived |
| Sahu et al. [15] (2019) | 1 | Ventilator-associated pneumonia | Five-month-old baby with congenital heart disease | Cotrimoxazole in combination with ciprofloxacin | Died |
| Raghavan et al. [16] (2017) | 1 | Urinary tract infection | Congestive heart failure | Minocycline | Survived |
| Dziuban et al. [17] (2018) | 283 Pediatric cases from 28 countries (1944–2017) | Spine was the most common site of infection. | Infants | Treatment details and susceptibility patterns not available | 138 Survived; 89 dieda |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download