This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Evidence for using high-flow nasal cannula (HFNC) in hypercapnia is still limited. Most of the clinical studies had been conducted retrospectively, and there had been conflicting reports for the effects of HFNC on hypercapnia correction in prospective studies. Therefore, more evidence is needed to understand the effect of the HFNC in hypercapnia.
Methods
We conducted a multicenter prospective observational study after applying HFNC to 45 hospitalized subjects who had moderate hypercapnia (arterial partial pressure of carbon dioxide [PaCO2], 43–70 mm Hg) without severe respiratory acidosis (pH <7.30). The primary outcome was a change in PaCO2 level in the first 24 hours of HFNC use. The secondary outcomes were changes in other parameters of arterial blood gas analysis, changes in respiration rates, and clinical outcomes.
Results
There was a significant decrease in PaCO2 in the first hour of HFNC application (-3.80 mm Hg; 95% confidence interval, -6.35 to -1.24; P<0.001). Reduction of PaCO2 was more prominent in subjects who did not have underlying obstructive lung disease. There was a correction in pH, but no significant changes in respiratory rate, bicarbonate, and arterial partial pressure of oxygen/fraction of inspired oxygen ratio. Mechanical ventilation was not required for 93.3% (42/45) of our study population.
Conclusions
We suggest that HFNC could be a safe alternative for oxygen delivery in hypercapnia patients who do not need immediate mechanical ventilation. With HFNC oxygenation, correction of hypercapnia could be expected, especially in patients who do not have obstructive lung diseases.
Go to :

Keywords: carbon dioxide, hypercapnia, mechanical ventilation, nasal cannula
INTRODUCTION
High-flow nasal cannula (HFNC) is a relatively new device for oxygenation that has become a popular tool in the treatment of hypoxic respiratory failure [
1]. Recently, there has been a growing interest in its use in hypercapnia. Physiological studies have shown that HFNC reduces rebreathing of carbon dioxide in the upper and lower anatomical dead space [
2,
3]. HFNC users generate less carbon dioxide by reduced work of breathing [
4]. Some studies have also suggested that HFNC may even improve alveolar ventilation [
5]. However, the effectiveness of HFNC in hypercapnic respiratory failure has not been fully demonstrated as relevant studies have been mostly retrospective with some contradictory results [
6-
8].
To clearly understand the effects of HFNC in hypercapnic respiratory failure, we conducted a prospective observational study with frequent arterial blood gas analysis (ABGA) in the first 24 hours after starting HFNC. For safety reasons, we recruited patients with mild to moderate hypercapnia without severe acidosis. The aim of this study was to examine the competence of HFNC in hypercapnia correction and to investigate the clinical use of HFNC in patients with hypercapnia.
Go to :

MATERIALS AND METHODS
A multicenter prospective observational study was conducted in four tertiary care hospitals in South Korea from November 2017 to October 2018. Inclusion criteria for the subjects were patients with mild to moderate hypercapnia (43 ≤ arterial partial pressure of carbon dioxide [PaCO2] ≤ 70 mm Hg) in ABGA. Recruitment was conducted from the emergency room, general ward, and intensive care unit. Exclusion criteria were: under 18 years of age; respiratory rate more than 35 breaths per minute; severe acidosis (pH < 7.30); using accessory muscle of respiration; any other signs of progressive respiratory failure requiring imminent intubation and mechanical ventilation; unstable hemodynamics; post-cardiopulmonary resuscitation status or on extracorporeal membrane oxygenation; consciousness disorder that would require intubation (e.g., not responsive, agitated, uncooperative, high risk for aspiration); facial surgery, trauma or deformity and airway obstruction that precluded application of nasal cannula; and those receiving end-of-life care.
HFNC was initiated with a pulse oximeter oxygen saturation target of 92%. The initial flow was set at 35 L/min and adjusted to suit the best comfort of the subject. ABGA was checked at 1, 3, 6, and 24 hours after starting HFNC. When the 24-hour data collecting period ended, the attending physician decided on the continuation and duration of the HFNC. Medications such as bronchodilators and antibiotics were administered to treat the underlying medical condition and precipitating factors.
HFNC was discontinued if the subject met any of the following criteria: symptoms and signs of worsening respiratory distress, such as retractions or sweating; increased respiratory rate to more than 35 breaths per minute; elevation of PaCO2 greater than 10 mm Hg from the previous ABGA results; pH less than 7.2 in any of the ABGA results; pH decrease more than 0.2 from previous ABGA results; or deteriorating mental status.
Study Outcomes
The primary outcome was change in ABGA parameters during the first 24 hours of HFNC application. We also analyzed change in PaCO2 based on underlying lung conditions. We hypothesized that the reduction in PaCO2 would be greater in patients with an obstructive pulmonary pathology. The secondary outcome was the clinical course of the subjects during hospitalization, which included discontinuation of HFNC according to the criteria, progression to invasive or noninvasive mechanical ventilation, and death.
Statistical Analysis
One-way repeated measure analysis of variance with a post hoc test by Bonferroni correction was used to evaluate the changes in ABGA parameters. Descriptive statistics were used for the clinical courses of subjects. The level of significance was set at 0.05. IBM SPSS ver. 25 (IBM Corp., Armonk, NY, USA) was used for all analyses.
Ethics Statement
The Institutional Review Boards of each participating hospital approved the study and written informed consent was obtained from all study participants prior to initial observation. This study is registered in the Korean Clinical Research Information Service (CRIS,
https://cris.nih.go.kr; registration No. KCT0003448).
Go to :

RESULTS
Study Population
A total of 51 subjects were recruited for the study. Six subjects dropped out of the study due to discomfort associated with HFNC (
Figure 1). The general characteristics of the 45 study subjects are reported (
Table 1). The most common acute lung condition was pneumonia followed by pulmonary edema and chronic obstructive pulmonary disease (COPD) exacerbation. In total, 87% (39/45) of the patients were receiving conventional oxygen therapy at the time of enrolment. For the initial HFNC settings, the median fraction of inspired oxygen (FiO
2) provided was 32% with a median flow of 35 L/min.
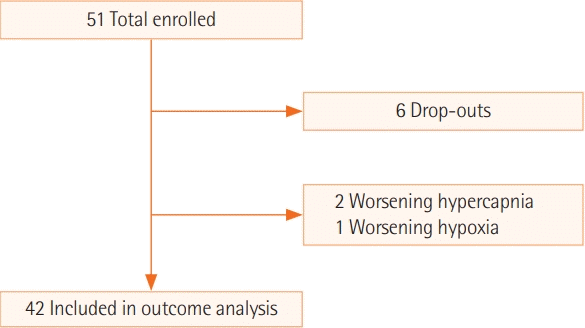 | Figure 1. Flow of study participants. 
|
Table 1.
General characteristics of enrolled subjects
|
Characteristics |
Total (n=45) |
|
Demographics |
|
|
Age (yr) |
70 (62–81) |
|
Male sex |
27 (60.0) |
|
BMI (kg/m2) |
21.3 (18.0–25.2) |
|
Acute lung condition |
|
|
Pneumonia |
23 (48.9) |
|
Pulmonary edema |
15 (33.3) |
|
COPD exacerbation |
12 (26.7) |
|
Atelectasis |
6 (13.3) |
|
Asthma exacerbation |
3 (6.7) |
|
Baseline conventional O2
|
|
|
Nasal prong |
32 (71.1) |
|
Face mask |
7 (15.6) |
|
Initial HFNC setting |
|
|
FiO2 (%) |
32 (25–37) |
|
Flow (L/min) |
35 (30–37.5) |

Primary Outcome
Changes in ABGA parameters are illustrated (
Figure 2). There were significant pH and PaCO
2 changes after HFNC application (
Table 2). For PaCO
2, a significant decrease was observed between the baseline and 1-hour measurement (–3.80 mm Hg; 95% confidence interval [CI], –6.35 to –1.24; P < 0.001). Among other checkpoints, there were no significant differences. Similarly, for pH, a significant increase was observed between the baseline and 1-hour measurement (0.030; 95% CI, 0.050–0.010; p < 0.001). In other parameters such as arterial bicarbonate concentration (HCO
3) and arterial partial pressure of oxygen/fraction of inspired oxygen ratio, no significant differences were observed between checkpoints.
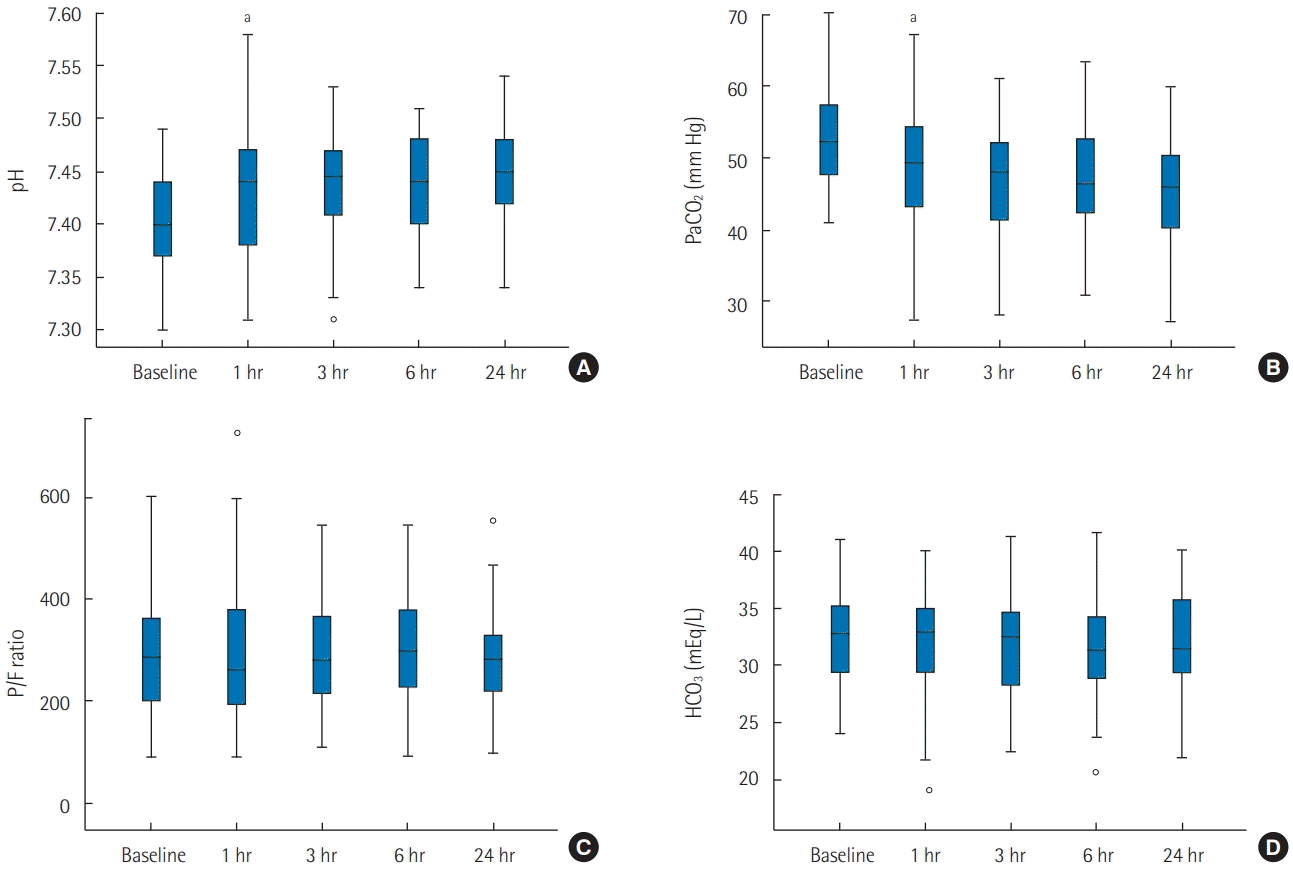 | Figure 2.Change in arterial blood gas analysis parameters at baseline, 1, 3, 6, and 24 hours after applying high-flow nasal cannula. (A) pH. (B) Arterial partial pressure of carbon dioxide (PaCO2). (C) Arterial partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2) ratio (P/F ratio). (D) Arterial bicarbonate concentration (HCO3). aStatistically significant change from previous measurement. 
|
Table 2.
Changes in parameters of arterial blood gas analysis after high-flow nasal cannula application
|
Variable |
Baseline |
1 hr |
3 hr |
6 hr |
24 hr |
P-value |
|
pH |
7.402±0.05 |
7.431±0.06 |
7.436±0.05 |
7.435±0.05 |
7.444±0.05 |
<0.001 |
|
PaCO2 (mm Hg) |
52.8±6.8 |
49.0±9.0 |
47.6±7.0 |
46.9±7.0 |
45.9±8.3 |
<0.001 |
|
P/F ratio |
99±93 |
294±134 |
288±108 |
296±105 |
283±96 |
0.826 |
|
HCO3 (mEq/L) |
31.8±5.3 |
32.2±5.0 |
32.0±4.4 |
31.6±4.5 |
31.4±4.5 |
0.518 |

PaCO2 changes in the obstructive and non-obstructive groups
We analyzed changes in PaCO2 after grouping the subjects based on underlying lung diseases. The “obstructive group” included subjects who had previously been diagnosed with obstructive lung disease: COPD, chronic bronchitis, emphysema, bronchiectasis and asthma. The “non-obstructive group” included subjects who had no documented obstructive lung disease. There were 18 subjects in the obstructive group and 24 in the non-obstructive group. Between the two groups, there was no statistical difference in age, sex, BMI, baseline ABGA results, and the initial HFNC setting.
PaCO
2 changes in separate groups are illustrated (
Figure 3). In the obstructive group, mean PaCO
2 decreased from 54.2 to 51.8, 49.7, 49.6, and 48.3 mm Hg. These changes were not statistically significant (P=0.075). However, a significant decrease of PaCO
2 was also found in the non-obstructive group. As mean PaCO
2 decreased from 51.7 to 46.9, 46.0, 44.8, and 44.2 mm Hg, there was a significant decrease between baseline and 1-hour measurement (–4.83 mm Hg; 95% CI, –8.4 to –1.3; P < 0.001).
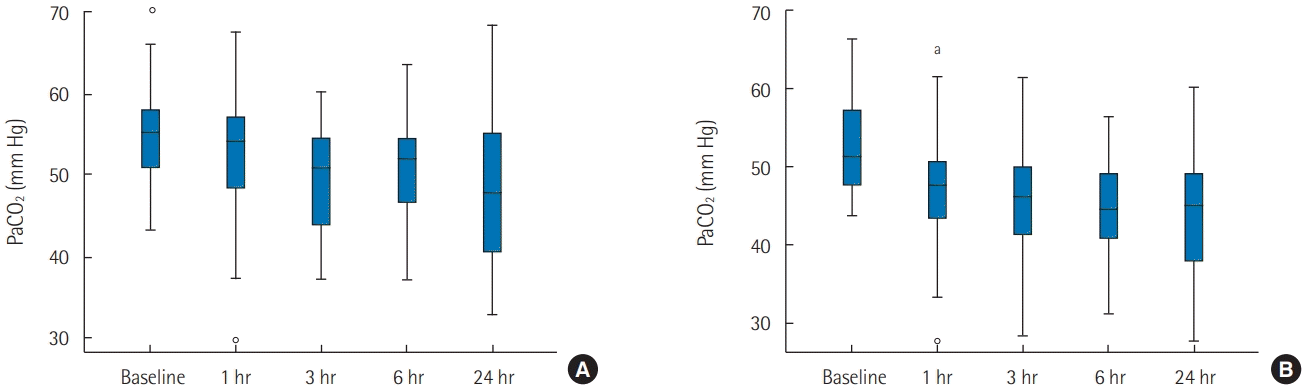 | Figure 3.Change in arterial partial pressure of carbon dioxide (PaCO2) at baseline, 1, 3, 6, and 24 hours after applying high-flow nasal cannula. (A) Obstructive group. (B) Non-obstructive group. aStatistically significant change from previous measurement. 
|
Secondary Outcome
Among the 45 study subjects, three subjects (6.7%) met the criteria for HFNC discontinuation. Two patients had an increase in PaCO2 of more than 10 mm Hg, and one patient showed symptoms and signs of worsening respiratory distress. Noninvasive ventilation was applied to the two patients with worsening hypercapnia. Invasive mechanical ventilation was applied to the patient with worsening respiratory distress. For the remainder of the 42 subjects, HFNC was continued for an average of 47 hours (median, 33 hours; interquartile range, 24–70 hours). No deaths were observed among the study population.
Go to :

DISCUSSION
In this prospective observational study, we report our findings on changes in ABGA parameters after applying HFNC to patients with mild to moderate hypercapnia. A significant decrease in PaCO2 and pH correction were observed in the first hour of HFNC application. The effect of PaCO2 reduction was more prominent in patients who did not have underlying obstructive lung disease. In terms of clinical outcomes of the 45 study subjects, two subjects required noninvasive mechanical ventilation for worsening hypercapnia, and one subject required invasive mechanical ventilation after intubation for worsening respiratory distress. There were no deaths or serious adverse outcomes in the study population.
With its unique physiological benefits, HFNC has become a popular device for oxygen delivery. HFNC provides a constant FiO
2, improves mucociliary clearance, reduces the effort required to breathe, and decreases anatomical dead space [
1]. With acute hypoxic respiratory failure, multiple prospective randomized trials have shown the clinical advantages of HFNC over conventional oxygen delivery systems [
9,
10]. Therefore, HFNC is widely being used in various conditions with hypoxia.
However, the effects of HFNC on hypercapnic respiratory failure remain unclear; therefore, more evidence is needed to support its use in patients with hypercapnia. Although most clinical studies have reported a reduction in PaCO
2 after applying HFN, there have been some studies with conflicting results. In a study by Sztrymf et al. [
8], involving intensive care unit patients with acute respiratory failure, a significant increase in PaCO
2 was observed. In a study by Nilius et al. [
11], the use of HFNC resulted in different individual responses in respiratory rates and PaCO
2 levels.
Because hypercapnia has complex pathophysiological mechanisms and multiple etiologies, there is wide heterogeneity in patients [
12]. This could explain the mixed results in clinical studies and becomes a challenge to establishing clear indications for the use of HFNC in hypercapnic respiratory failure. However, even with various causes, there are commonalities in these patients. Whatever the diagnosis, mechanical ventilation (invasive and noninvasive) is a standard treatment in severe cases of hypercapnia [
13]. Therefore, the potential CO
2 removal capacity and physiologic benefits make HFNC an attractive alternative to conventional oxygen therapy in patients with hypercapnia.
With our study, an overall decrease in PaCO
2 with a correction of pH was observed after applying HFNC in patients with mild to moderate hypercapnia. Additionally, 93% of the study population was successfully treated without progressing to mechanical ventilation. In other studies investigating clinical implications of HFNC on hypercapnic respiratory failure, intubation rate ranged from 0% to 25% [
14,
15]. A recent metaanalysis suggested that HFNC was not inferior to noninvasive ventilation with respect to intubation rate and mortality [
16]. Considering all current evidence, HFNC may be a feasible alternative option for oxygen supplementation in patients with mild to moderate hypercapnia.
However, our study found a different response to HFNC depending on underlying lung disease. We analyzed changes in PaCO2 after grouping the patients according to the presence or absence of an obstructive lung pathology. Contrary to our anticipation, the effects of HFNC were more pronounced in patients who did not have underlying obstructive lung disease. This finding suggests that the decrease in PaCO2 during HFNC was possibly related to the decreased effort required to breathe rather than reduction in dead space. Further studies are needed to better understand the effects of HFNC on hypercapnia based on underlying lung pathology.
We recognize the limitations of this study. We acknowledge that the findings in this study cannot be fully accepted without a control group comparison. Also, because of the nature of this study design, selection bias is possible. Last, the small number of subjects also limits the ability to generalize the study’s findings.
In conclusion, we prospectively investigated changes in ABGA parameters and clinical implications of HFNC in patients with mild to moderate hypercapnia. After applying HFNC, an overall decrease in PaCO2 with pH correction was seen. Most of our subjects were treated successfully without progressing to mechanical ventilation. Therefore, we suggest that HFNC could be a feasible alternative option for oxygen delivery in patients with mild to moderate hypercapnia. However, further controlled studies with different etiologies of hypercapnia are needed to establish the clear role of HFNC in patients with hypercapnic respiratory failure.
Go to :

KEY MESSAGES
■ When high-flow nasal cannula was applied in moderate hypercapnia patients without severe respiratory acidosis, reduction of carbon dioxide and correction of pH was seen.
■ Progression to mechanical ventilation was comparable to previous studies.
■ We suggest that high-flow nasal cannula could be a safe alternative for oxygen delivery in hypercapnia patients who do not need immediate mechanical ventilation.
Go to :






 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download