1. Kloppel G, Couvelard A, Hruban RH, Klimstra DS, Komminoth P, Osamura RY, et al. Introduction. In : Lloyd RV, Osamura RY, Kloppel G, Rosai J, editors. WHO classification of tumours of endocrine organs. 4th ed. Lyon: IARC Press;2017. p. 211–4.
2. Klimstra DS, Arnold R, Capella C, Hruban RH, Kloppel G, Komminoth P, et al. Neuroendocrine neoplasms of the pancreas. In : Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC Press;2010. p. 322–6.
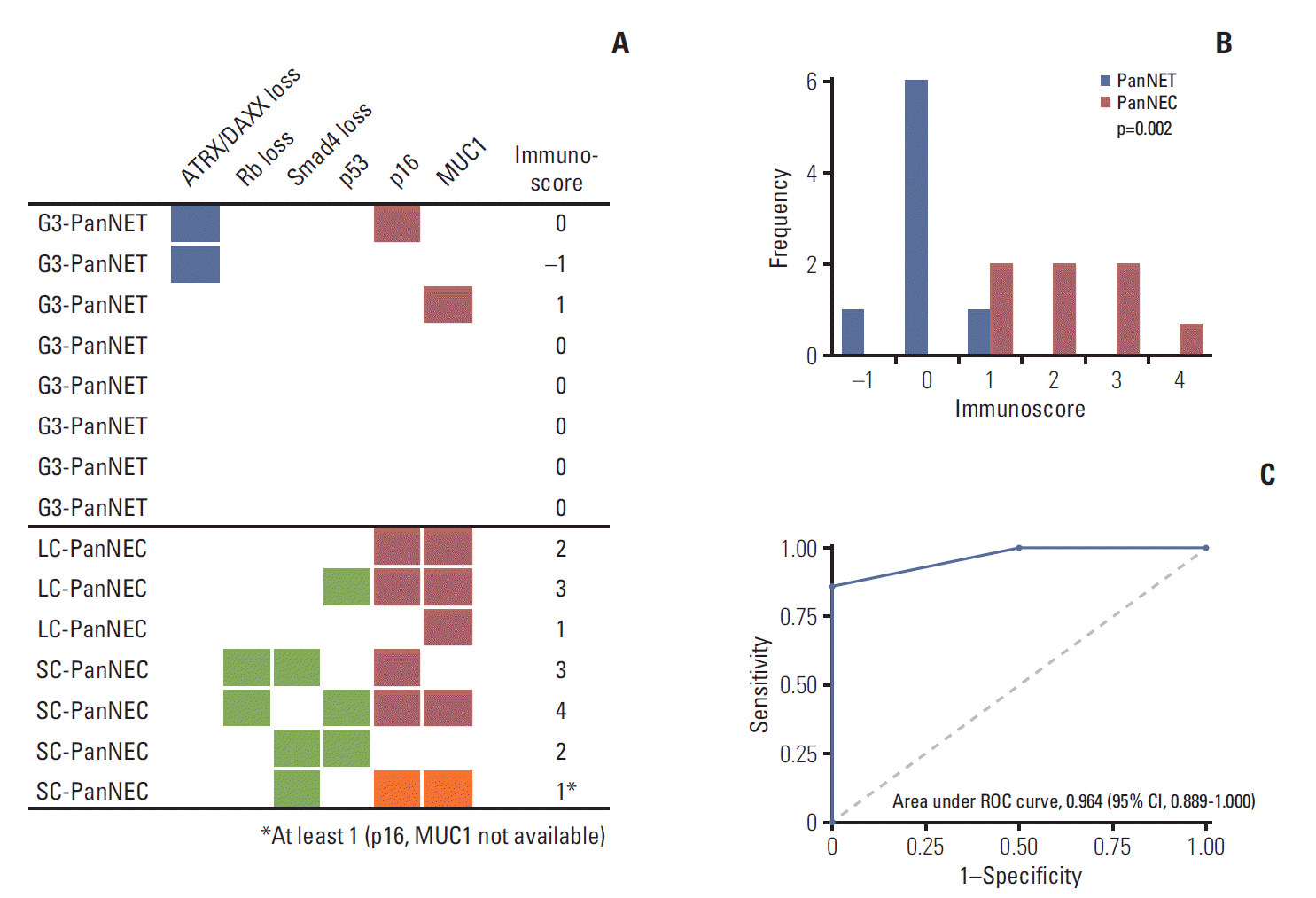
3. Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM, et al. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol. 2015; 39:683–90.

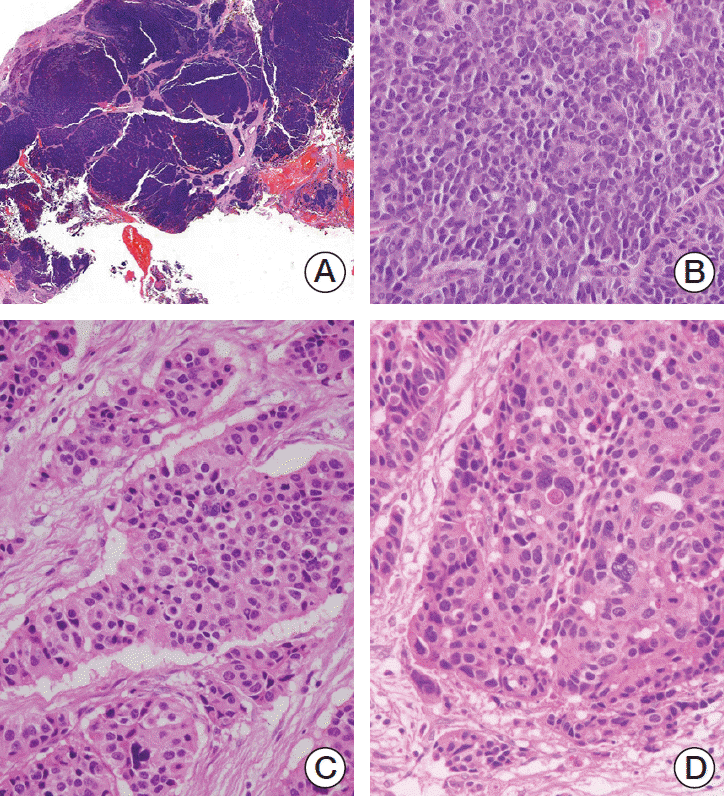
4. Tang LH, Untch BR, Reidy DL, O'Reilly E, Dhall D, Jih L, et al. Well-differentiated neuroendocrine tumors with a morphologically apparent high-grade component: a pathway distinct from poorly differentiated neuroendocrine carcinomas. Clin Cancer Res. 2016; 22:1011–7.

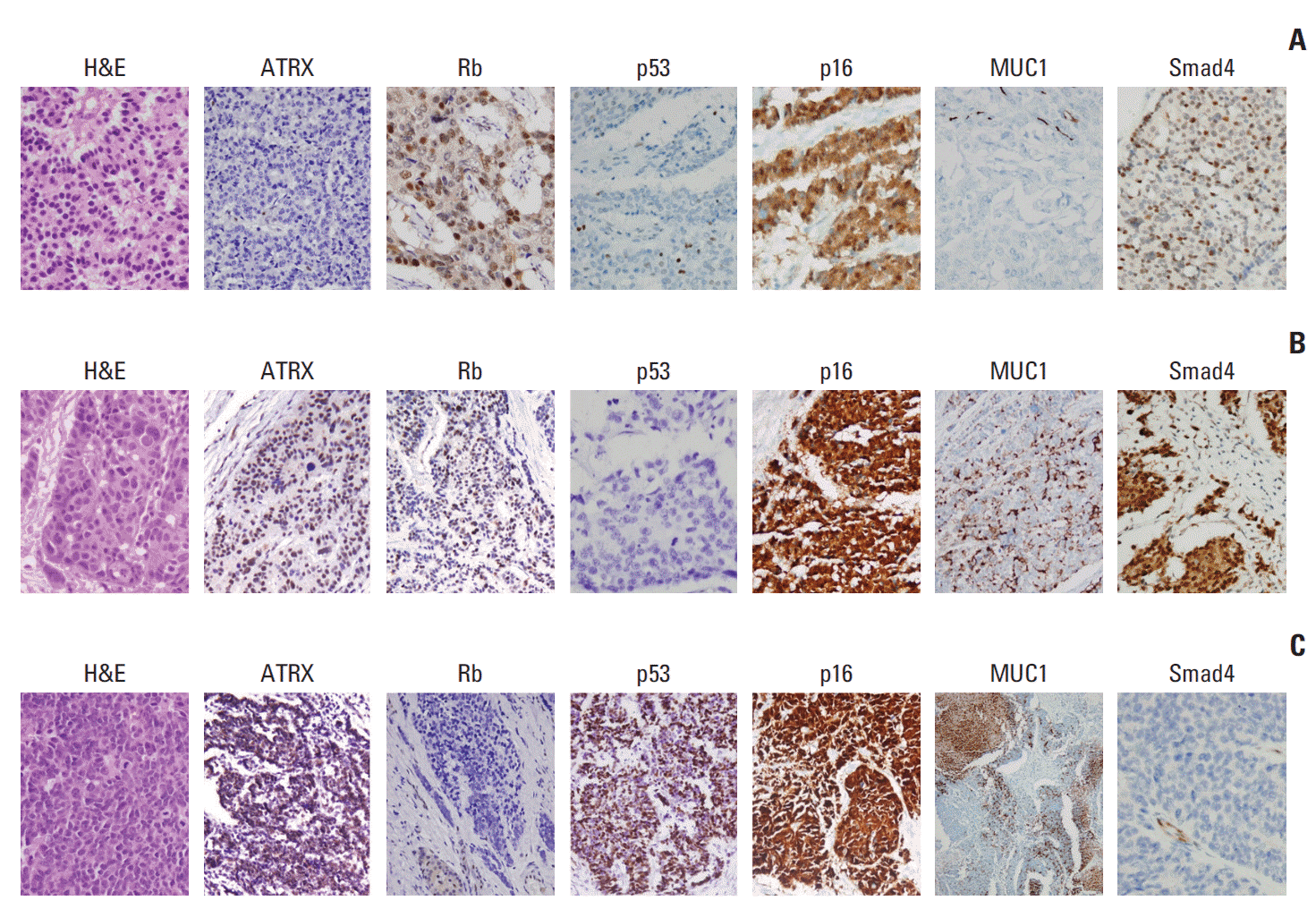
5. Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011; 331:1199–203.
6. Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012; 36:173–84.

7. Raj N, Valentino E, Capanu M, Tang LH, Basturk O, Untch BR, et al. Treatment response and outcomes of grade 3 pancreatic neuroendocrine neoplasms based on morphology: well differentiated versus poorly differentiated. Pancreas. 2017; 46:296–301.
8. Adsay NV, Klimstra DS, Kloppel G, Oberg K, Papotti M, Rindi G, et al. Pancreatic neuroendocrine carcinoma (poorly differentiated
neuroendocrine neoplasm). In : Lloyd RV, Osamura RY, Kloppel G, Rosai J, editors. WHO classification of tumours of endocrine organs. 4th ed. Lyon: IARC Press;2017. p. 235–7.
9. Heetfeld M, Chougnet CN, Olsen IH, Rinke A, Borbath I, Crespo G, et al. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2015; 22:657–64.

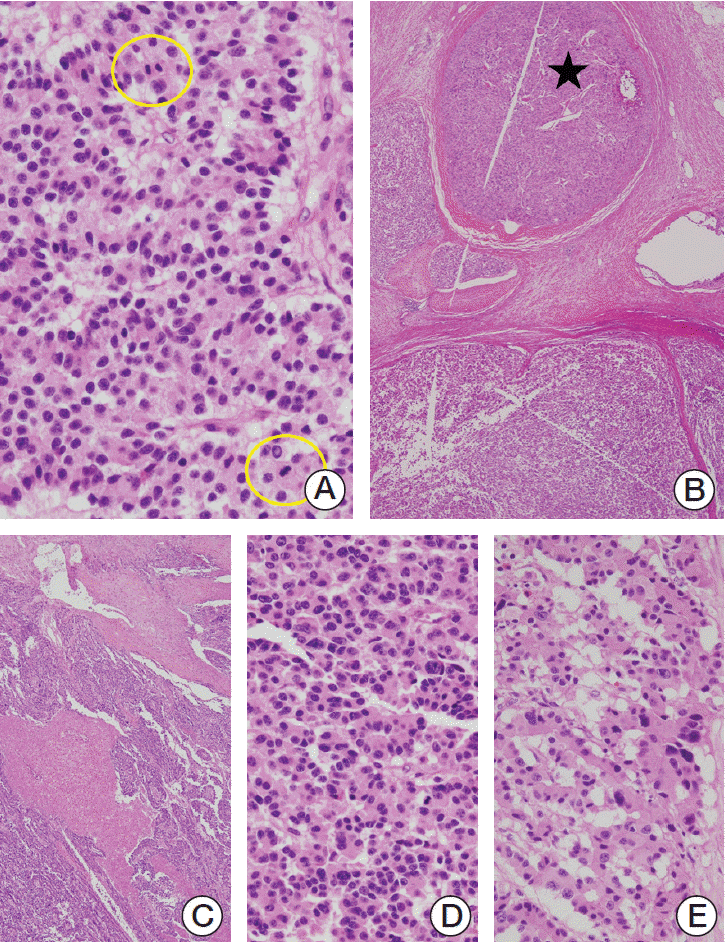
10. Zee SY, Hochwald SN, Conlon KC, Brennan MF, Klimstra DS. Pleomorphic pancreatic endocrine neoplasms: a variant commonly confused with adenocarcinoma. Am J Surg Pathol. 2005; 29:1194–200.
11. Konukiewitz B, Schlitter AM, Jesinghaus M, Pfister D, Steiger K, Segler A, et al. Somatostatin receptor expression related to TP53 and RB1 alterations in pancreatic and extrapancreatic neuroendocrine neoplasms with a Ki-67-index above 20. Mod Pathol. 2017; 30:587–98.

12. Konukiewitz B, Jesinghaus M, Steiger K, Schlitter AM, Kasajima A, Sipos B, et al. Pancreatic neuroendocrine carcinomas reveal a closer relationship to ductal adenocarcinomas than to neuroendocrine tumors G3. Hum Pathol. 2018; 77:70–9.

13. Kakar S, Pawlik TM, Allen PJ, Vauthey JN. Exocrine pancreas. In : Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, editors. AJCC cancer staging manual. 8th ed. Chicago, IL: Springer;2017. p. 337–47.
14. Hwang HS, Kim Y, An S, Kim SJ, Kim JY, Kim SY, et al. Grading by the Ki-67 labeling index of endoscopic ultrasoundguided fine needle aspiration biopsy specimens of pancreatic neuroendocrine tumors can be underestimated. Pancreas. 2018; 47:1296–303.

15. Tang LH, Basturk O, Sue JJ, Klimstra DS. A practical approach to the classification of WHO grade 3 (G3) well-differentiated neuroendocrine tumor (WD-NET) and poorly differentiated neuroendocrine carcinoma (PD-NEC) of the pancreas. Am J Surg Pathol. 2016; 40:1192–202.

16. Kim JY, Brosnan-Cashman JA, An S, Kim SJ, Song KB, Kim MS, et al. Alternative lengthening of telomeres in primary pancreatic neuroendocrine tumors is associated with aggressive clinical behavior and poor survival. Clin Cancer Res. 2017; 23:1598–606.

17. Kim JY, Lee SH, An S, Kim SJ, Sung YN, Song KB, et al. Carbonic anhydrase 9 expression in well-differentiated pancreatic neuroendocrine neoplasms might be associated with aggressive behavior and poor survival. Virchows Arch. 2018; 472:739–48.

18. Kim SJ, An S, Lee JH, Kim JY, Song KB, Hwang DW, et al. Loss of progesterone receptor expression is an early tumorigenesis event associated with tumor progression and shorter survival in pancreatic neuroendocrine tumor patients. J Pathol Transl Med. 2017; 51:388–95.

19. Grillo F, Bruzzone M, Pigozzi S, Prosapio S, Migliora P, Fiocca R, et al. Immunohistochemistry on old archival paraffin blocks: is there an expiry date? J Clin Pathol. 2017; 70:988–93.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download