Introduction
Prostate cancer (PCa) is the second most common cancer worldwide and most common urological cancer in South Korea [
1]. It is mainly prevalent in the elderly. Over 70% of PCa patients are over 65 years old [
2]. In South Korea, the incidence of PCa has increased rapidly from about 1,500 cases in 1999 to about 9,000 cases in 2011. Age-standardized incidence rates of PCa had also increased from about 9 per 100,000 in 1999 to over 25 per 100,000 in 2011. In patients over 65 years old, PCa incidence has continuously increased. Nearly 80% of PCa patients were over 65 years old in 2014. The incidence and prevalence of PCa have continuously increased [
3,
4].
South Korea has become an aging society rapidly in recent years [
5]. This is the biggest issue in social, economic, and medical areas. Considering that PCa is mainly diagnosed at old age, PCa is associated with aging. Hence, PCa incidence and prevalence could be affected by the aging phenomenon. PCa incidence according to age showed an increasing trend from age group of over 50s and peaked in age group of 70s in South Korea [
3]. Interestingly, it has a similar trend in United States [
5]. Almost 95% of PCa patients are over 50s. The most frequent age group of PCa patients is age group of 60s, with almost 45% of total PCa patients [
5]. PCa patients aged over 70s accounted for about 30% of total PCa patients in the United States [
6].
Since the 1990s, serum prostate-specific antigen (PSA) has been used for screening test of PCa in the United States. After using PSA, the incidence was doubled in 1992 compared to that in 1986 [
7]. According to the U.S. Preventive Services Task Force (USPSTF) in 2008, PCa screening using PSA was not recommended for those over 75 years. After announcement of USPSTF recommendation in 2008, PCa incidence decreased from 2010 to 2012 in the United States [
7].
Moreover, recent studies are showing a new phenomenon that newly detected PCa has more aggressive pattern including high Gleason`s score and increased metastatic state. It is currently unclear whether this phenomenon is affected by decreasing PCa screening using PSA or other reasons [
8,
9]. Hence, the recommendation was changed in 2017, suggesting that individuals aged 55 to 69 years should be tested based on their own decision with their urologist. However, those aged over 70 years are not recommended to be tested. Thus, the new recommendation encourage PSA screening test more positively for those under 70 years compared to USPSTF recommendations in 2012 [
10].
Similar to United States, incidence of PCa in Korea could be affected by many factors such as change of national medical policy and population structural change. The goal of this study was to investigate the trend of PCa incidence between 2003 and 2013, focusing on period to determine whether there might be a period effect of a specific year. Effects of age, period, and birth cohort on PCa incidence were analyzed using age-period-cohort analysis.
Go to :

Materials and Methods
1. Data collection
Annual report of cancer statistics between 2003 and 2013 from National Health Insurance Service (NHIS) in South Korea for the number of PCa patients and Korean Statistical Information Service (KOSIS) data between 2003 and 2013 from national statistics in South Korea for the number of Korean male population were used. The NHIS which covers 99% of all Koreans provides national medical insurance and collects data, including the total number of patients for each cancer, age, sex, diagnosis, and so on. The NHIS database also includes disease diagnosis and medical procedure using disease code because almost all payments are based on feefor-service. PCa (C61) was coded according to the Korean standard classification of disease and cause of death, 6th edition (KCD-6). KOSIS collects every field of data. Such data are collected by public organization in South Korea led by government to provide statistics for public or private research.
2. Data analysis
Age-standardized PCa incidence rates per 100,000 were calculated from 2003 to 2013. Age groups for those aged 40 years and older were subdivided at 5-year intervals. Annual percentage change (APC) was calculated after three years from 2011 that predicted 2014 data.
We performed age-period-cohort analysis to evaluate effects of age, period, and birth cohort. Both age (from 40 years old) and period (from 2003 to 2013) were subdivided by 5-year intervals. Birth cohorts began in 1918 and ended in 2008 using U.S. National Cancer Institute web-based statistical tool proposed by Rosenberg et al. [
11]. The result of age-period-cohort analysis included PCa incidence according to age, period, and birth cohort, distribution of PCa incidence within age, period and birth cohort, respectively, according to other two indexes and age-period-cohort effects on PCa incidence.
Age-standardized rates of PCa incidence were adjusted based on residence-registration mid-year population in 2010. APCs of PCa incidence age-standardized rate were calculated during study period and 95% confidential interval for APC was calculated.
In age-period-cohort analysis, because of co-linearity that the birth cohort corresponds to the difference between period (year of diagnosis) and age at the time of diagnosis (cohort= period–age), it cannot be assumed as effect of three indexes at the same time. Thus, we used intrinsic estimator using apc.fit of Epi package in R (v3.1.2, R Foundation, Vienna, Austria) to solve specification of age-period-cohort model. The likelihood ratio test compared to goodness-of-fit of models measured by deviance and degrees of freedom. We used equation in regression model for age-period-cohort analysis that log[λ(a,p)]=ƒ(a)+g(b)+h(c). Findings at p < 0.05 were considered significant.
3. Ethical statement
The study protocol was approved by the Institutional Review Board of Soonchunhyang University Hospital (2019-07-016). Informed consent requirements were waived because the study was based on routinely collected administrative data and patient data were kept anonymous.
Go to :

Results
Table 1 and
Fig. 1 show age-standardized PCa incidence rate and APC in South Korea males from 2003 to 2013. Total APC of PCa incidence was 8.8% (p < 0.001; 95% confidential interval, 6.5 to 11.2). It showed an increasing pattern from 2003 to 2001 but a decreasing pattern from 2011 to 2013. PCa incidence was significyantly increased in those over 45 years old from 2003 to 2013. Each age group under 75 years old showed nearly 10% of APC (p < 0.001 and p=0.011 for over 85 years, respectively). Total APC of PCa incidence for age proups over 60 years old also showed an increasing pattern from 2003 to 2011 and a decreasing pattern from 2011 to 2013.
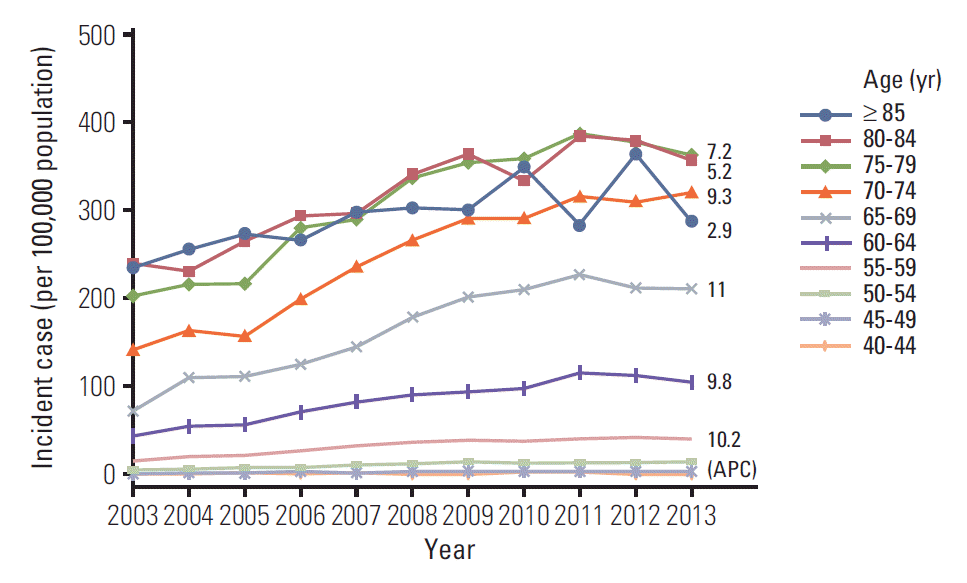 | Fig. 1.Age-standardized rates of prostate cancer incidence in South Korean males, 2003-2013. 
|
Table 1.
Age-standardized rates of PCa incidence in South Korean males, 2003-2013
|
Age group (yr) |
Patients with prostate cancera) (cases per 100,000)
|
|
2003 |
2004 |
2005 |
2006 |
2007 |
2008 |
2009 |
2010 |
2011 |
2012 |
2013 |
APC |
95% CI |
p-value |
|
40-44 |
0.3 |
0.4 |
0.7 |
0.4 |
0.7 |
0.6 |
0.4 |
1.0 |
0.9 |
0.5 |
0.6 |
6.2 |
–1.2 to 14.1 |
0.093 |
|
45-49 |
1.1 |
0.8 |
1.3 |
2.4 |
2.0 |
2.8 |
2.8 |
3.1 |
3.2 |
3.2 |
2.9 |
13.1 |
7.1 to 19.4 |
0.001 |
|
50-54 |
3.5 |
5.4 |
6.8 |
7.4 |
9.2 |
11.0 |
13.1 |
12.5 |
12.6 |
12.8 |
14.1 |
13.2 |
8.9 to 17.7 |
< 0.001 |
|
55-59 |
15.2 |
19.9 |
20.8 |
26.2 |
31.1 |
35.6 |
38.4 |
37.8 |
40.4 |
41.3 |
39.6 |
10.2 |
7 to 13.5 |
< 0.001 |
|
60-64 |
43.1 |
54.4 |
55.1 |
70.0 |
81.3 |
90.0 |
92.2 |
97.3 |
114.7 |
112.1 |
103.9 |
9.8 |
7.2 to 12.5 |
< 0.001 |
|
65-69 |
72.2 |
108.9 |
109.8 |
124.5 |
144.0 |
178.4 |
199.7 |
208.1 |
227.2 |
211.6 |
209.8 |
11 |
7.7 to 14.5 |
< 0.001 |
|
70-74 |
140.8 |
162.4 |
156.0 |
197.6 |
235.0 |
265.3 |
290.0 |
289.9 |
315.2 |
308.6 |
318.9 |
9.3 |
6.9 to 11.6 |
< 0.001 |
|
75-79 |
202.0 |
214.8 |
216.0 |
278.4 |
289.3 |
336.2 |
354.1 |
357.4 |
386.7 |
377.3 |
361.8 |
7.2 |
5 to 9.3 |
< 0.001 |
|
80-84 |
239.2 |
230.0 |
263.6 |
293.1 |
296.1 |
341.0 |
363.7 |
332.6 |
383.3 |
379.3 |
356.6 |
5.2 |
3.5 to 6.9 |
< 0.001 |
|
≥ 85 |
234.3 |
254.6 |
271.6 |
264.8 |
297.6 |
302.2 |
299.1 |
348.7 |
282.8 |
363.8 |
286.2 |
2.9 |
0.8 to 4.9 |
0.011 |
|
Totalb)
|
33.5 |
40.1 |
41.1 |
49.7 |
56.1 |
64.8 |
69.8 |
71.0 |
77.3 |
75.8 |
73.9 |
8.8 |
6.5 to 11.2 |
< 0.001 |

Table 2 shows goodness-of-fit test for age-period-cohort model. The age-period-cohort model was found to be the best model to explain the data (deviance for age-period-cohort model: 67.6 degree of freedom: 18; deviance for age-period-cohort model: 377.33, degree of freedom: 19; deviance for age-period-cohort: 171.42, degree of freedom: 22; deviance for age model: 1,697.1, degree of freedom: 24).
Table 2.
Goodness-of-fit of age-period-cohort model assessment for prostate cancer incidence rates in Korea, 2003-2013
|
Model |
DEV |
d.f. |
ΔDEV |
Δd.f. |
|
Apc |
67.6 |
18 |
Ref. |
Ref. |
|
Ac |
377.33 |
19 |
309.73 |
1 |
|
Ap |
171.42 |
22 |
103.82 |
4 |
|
Age |
1,697.1 |
24 |
1,629.5 |
6 |

Fig. 2 shows PCa incidence according to age/period/cohort indexes in South Korea males from 2003 to 2013 and age-period-cohort effects on PCa incidence in South Korea males from 2003 to 2013 using a multiple regression model. PCa incidence was significantly increased from age 45, peaked in age 70, and decreased at age 75 and over. In period indexe, PCa incidence had increased from 2003 to 2013, although its increasing speed was slowed down from 2008. IN cohort index, PCa incidence peaked in 1940s age cohort but decreased from the 1950 cohort. Age after adjustment of period and cohort indexes increased PCa incidence. However, the increasing was getting slower with age. After adjusting for age and cohort indexes, effect of period on PCa incidence showed a plateau pattern. PCa incidence was increased 1.4 times in 2008 compared to that in 2003 or 2013. After adjustment of age and period indexes, effect of cohort on PCa incidence started to increase. The risk of PCa incidence increased from 1958 cohort. It was also increased with recent cohort.
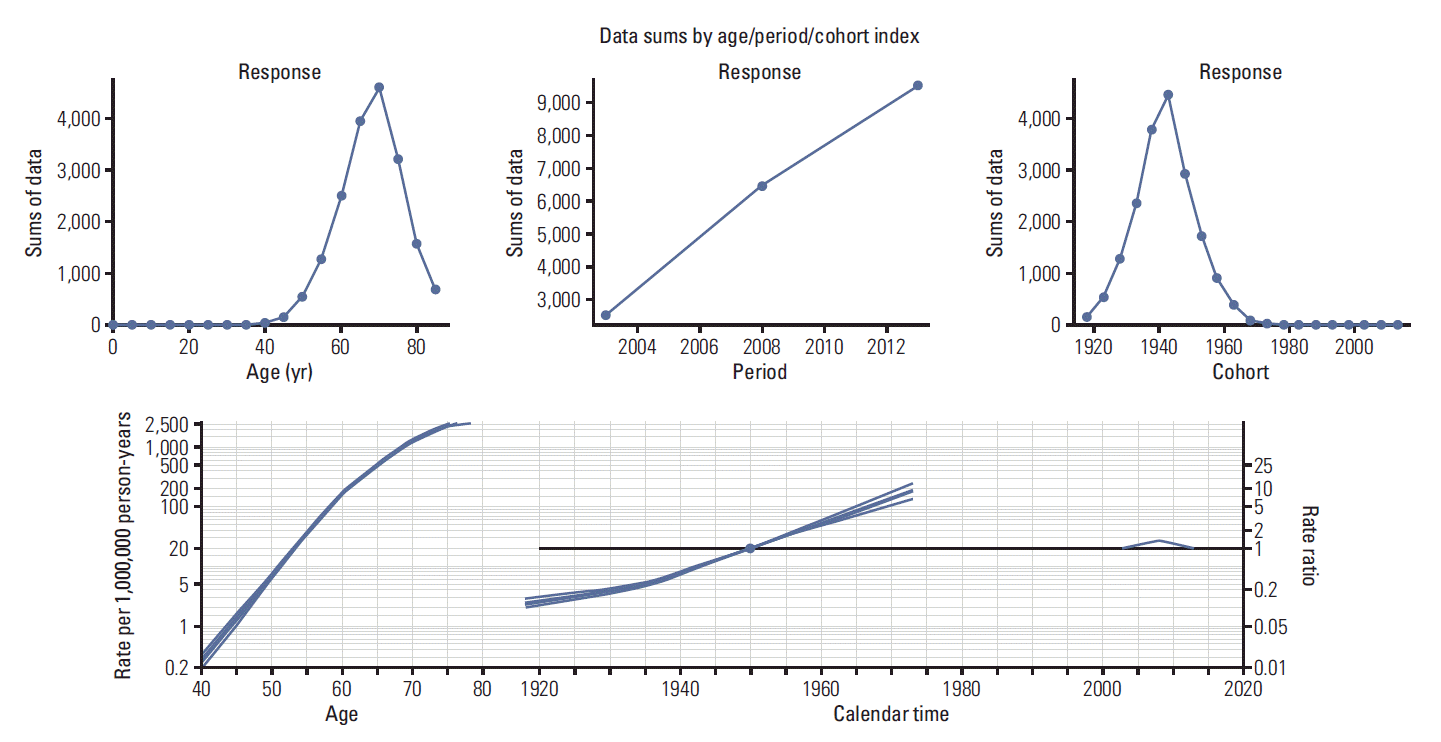 | Fig. 2.Prostate cancer (PCa) incidence according to age/period/cohort index in Korean males from 2003 to 2013 and age-period-cohort effects on PCa incidence in Korean males from 2003 to 2013. 
|
Fig. 3 shows distribution of PCa incidence in South Korea males from 2009 to 2013. In age-period graph, PCa incidence increased from 40s age group, peaked in 70s aged group, and decreased over 70s age groups in all period indexes, although change was more steep in recent period index. In age-cohort graph, PCa showed the highest incidence in 1938 cohort. Significant PCa incidence was also found in younger age with recent cohort. In cohort-period graph, high PCa incidence showed in 1938 and 1948 cohorts from 2003 to 2013. In period-age graph, PCa incidence showed an increasing speed was slowed down from 2008 to 2013 in ages over 50. In cohort-age graph, PCa incidence increased from age 40 and 1938, 1948, and 1958 cohorts showed higher PCa incidence compared to other cohorts at the same age. In period-cohort graph, there was nearly no PCa incidence before 1918 or after 1978 cohorts, showing an increasing pattern from 2003 to 2008 and a decreasing pattern from 2008 to 2013 in 1928 cohort. PCa incidence showed an increasing pattern, although its speed was slowed down from 2008 in 1928 and 1938 cohorts. It then showed an increasing pattern and its speed was faster from 2008 in 1948 and 1958 cohorts.
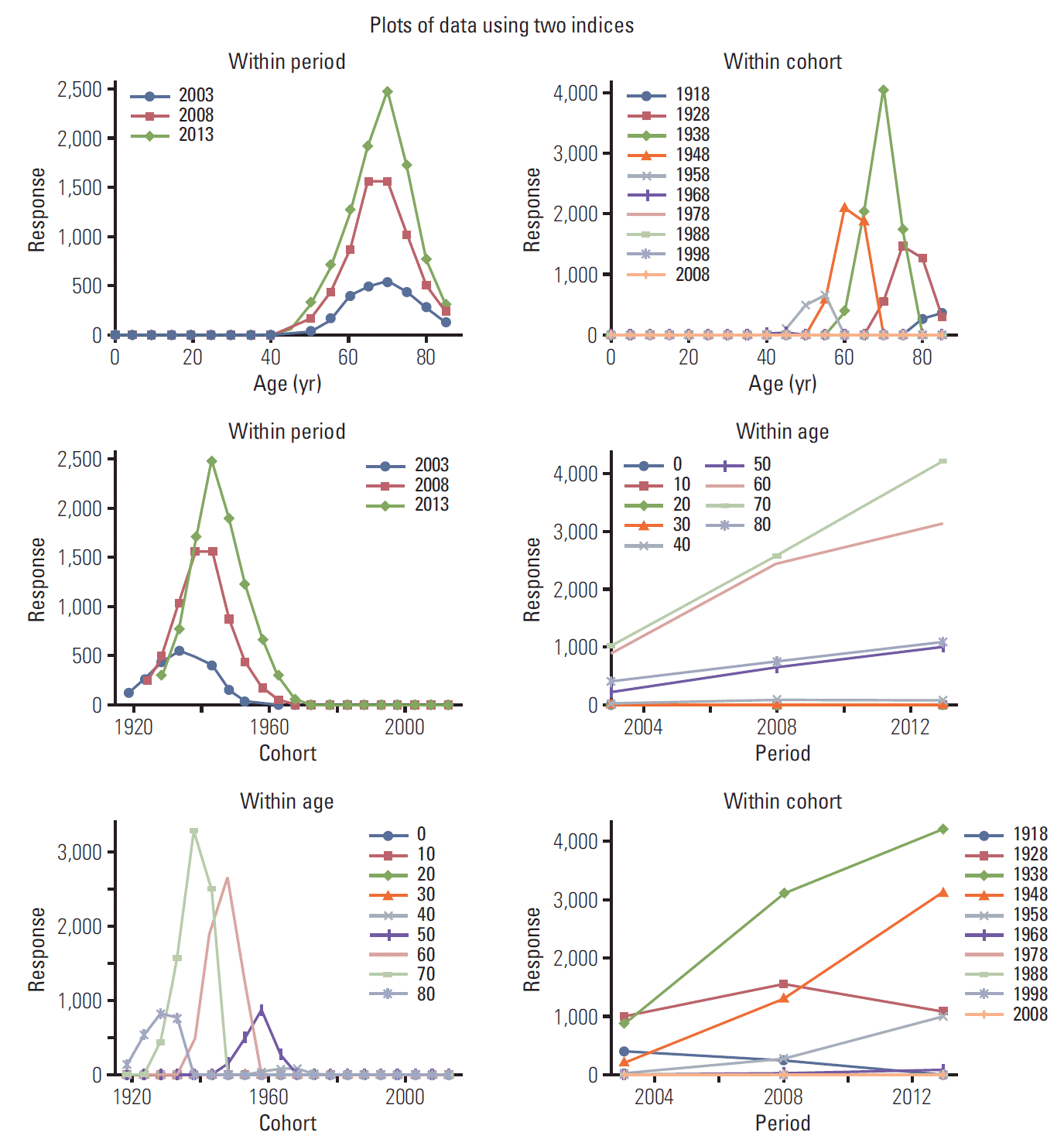 | Fig. 3.Distribution of prostate cancer incidence in Korean males from 2003 to 2013. 
|
Go to :

Discussion
This is the first study that investigates the incidence of PCa using age-period-cohort models in South Korea. Most of previous studies using age-period-cohort model are limited to mortality issue [
2,
12]. Our results showed that PCa incidence rate had an increasing trend from 2003 to 2011. It changed to show a decreasing trend from 2011 to 2013. Moreover, our data showed that there was a specific period effect of year by first time. The period effect was still significant after adjustment of age and period-cohort effect. Although this study did not provide direct evidence that screening pattern of South Korea was affected by USPSTF guideline of specific year, it provided indirect evidence of that possibility.
Incidence of PCa in South Korea was lower than that in other countries worldwide from 2000 to 2004 (9.5 per 100,000 in South Korea compared to 118.2 per 100,000 in the United States). However, it has increased rapidly among Asian countries, with APC of 13.8% from 1999 to 2007 [
13]. This increasing trend of PCa in developing countries might be explained by increasing portion of obesity population, decreased physical activity, and changed dietary distribution with increased portion of fat consumption [
14]. However, it may be also affected by imposition of PSA screening test for PCa and application of nationwide cancer registration system [
15].
Incidence of PCa has shown a marked increase during 1990s in most Western population after imposition of PSA screening for PCa. However, whether PSA screening could compensate the mortality rate of PCa ultimately remains controversial [
2]. After USPSTF recommendations in 2008 that encouraged those over 75 years not to perform routine PSA screening, PCa incidence rate started to decrease in the United States. USPSTF recommendation in 2012 announced that grade D (meaning no benefit over harm when procedure was done) for PCa screening using PSA in all male adults [
16]. Compared to recommendation in 2008, it becomes more powerful restriction for routine application of PSA screening for PCa.
Lin et al. [
17] have reported that there is no difference between PSA screening patients group and non-PSA screening patients group in total mortality or PCa specific mortality. However, there are more loses in that PSA screening patients group feels more negative psychological effects such as anxiety. This study supports the USPSTF recommendation for PSA screening test which is recognized as a routine PCa screening as group D considering overdiagnosis and overharm.
Barocas et al. [
6] have reported that PCa incidence is decreased about 30% in a year after USPSTF recommendation in 2008, especially a 40% decrease in low-risk PCa diagnosis. However, it could significantly affect Western male people, although it has relatively small effect on male South Korean because the percentage of those who took PSA screening test was only 15% in 2004 compared to 75% of total male populations who had taken PSA screening test in the United States [
18,
19].
In our results, the incidence of PCa had increased from 2003 to 2011 and decreased from 2011 to 2013. Because PCa incidence increased with age and recent cohort, it could explain the increasing trend of PCa incidence from 2003 to 2011. However, it could not explain the decreasing trend of PCa incidence from 2011 to 2013. Contrary to our null hypothesis that incidence of PCa in South Korea would be affected by Western guideline recommendations, there was a period effect which could explain direct or indirect effect of USPSTF recommendation in 2008.
Moreover, medical guidelines of South Korea have been affected by US guidelines in multiple medical departments [
20,
21]. For example, revised guidelines for thyroid nodules and cancers in South Korea were published in 2016 because American Thyroid Associations (ATA) guidelines in 2015 announced that fine-needle aspiration biopsy should be performed for thyroid nodules over 1 cm. Guidelines for hypertension in South Korea had also been affected by the 2003 Joint National Committee (JNC-7)’s new guidelines [
20,
21].
South Korea confronts the biggest social issue in that South Korea is becoming an aging society. It has the lowest fertility rate among Organization for Economic Cooperation and Development (OECD) countries. The proportion of Korea’s population over 65 years was about 7% in 2000 and 15% in 2015. It will increase to about 30% of the total population in South Korea by 2030 [
5].
Population structural change could affect increasing trend of PCa incidence from 2008 to 2011. However, it could not explain the decreasing trend. Age-period-cohort is a useful method to show whether there is an impact of aging phenomenon or period on specific incidence or mortality of disease. Age-period-cohort analysis can reveal the change of specific population composition, how period and cohort effect works, and socioeconomic, medical, biological factors that affect a specific population [
22].
It is known that if the cohort and period effect are not considered in analysis of the incidence of disease by age, there is a risk that the result is distorted [
23]. That is why APC analysis was performed in this study. Thus, it is reasonable to interpret using model 5, which considered the adjustment for cohort and period effects.
In addition, if the major interest is the pure effect of age on the incidence of PCa, which means the effect of the natural aging of the body, you can use the result of the adjusted model, whereas you want to figure out the incidence of PCa by age group in the real world where the cohort and period effects were mixed, then you can use the result of the unadjusted model.
With our results, it makes sense that the PCa incidence increases as the age increases from the adjusted model. But, when we considered the cohort effect corresponding to the variation of population structure, behavioral style, and social system, and the period effect related to the specific time simultaneously affecting all the members of the society, then the incidence of PCa showed the peaks at 70 years. Our results also provide useful information for the advantage of prostate cancer screening by nationwide level.
Bao et al. [
24] have reported major cancer incidence in urban Shanghai from 1973 to 2010 using age-period-cohort model. They also reported that PCa incidence in Shanghai had been dramatically increased in 40 years. Its APC was nearly 7% compared to approximately 12% in South Korea from 1999 to 2011 [
3]. The pattern of PCa incidence in Shanghai was similar to our result in that it showed a steep increase from 1989 to 2004 and then its speed was decreased from 2004 to 2010. They analyzed this phenomenon and found that cohort effect had increased continuously compared to period effect which remained steady during the study period because there was no national or regional PCa screening in Shanghai. PCa incidence was affected by social factors such as hospital accessibility.
This study has some limitations. First, although there was impact of Western PCa screening guideline on PCa incidence in South Korea, we explored age-period-cohort models to investigate whether there was an age or period effect. This does not provide direct evidence. Second, for age-periodcohort analysis, we assumed the result using intrinsic estimator because we could not calculate all three indices at the same time. The result is an estimate. It could be different from the real value. However, this is the best option to calculate the trend using age-period-cohort.
This study revealed that the trend of PCa incidence in South Korea is increasing from 2003 to 2011 and decreasing from 2011 to 2013. By age-period-cohort model, we could speculate that there was a positive cohort effect, meaning that PCa incidence was associated with age structural change. PCa incidence was also affected by a specific period.
Go to :





 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download