Introduction
Materials and Methods
1. Patients
Table 1.
| Variable |
Unmatched (complete) dataset |
Propensity score-matched (1:1) datasetb) |
||||||
|---|---|---|---|---|---|---|---|---|
| Total | Diabetic (n=73) | Non-diabetic (n=334) | p-value | Total | Diabetic (n=73) | Non-diabetic (n=73) | p-value | |
| Clinical variable | ||||||||
| Sex | ||||||||
| Male | 254 | 56 (76.7) | 198 (59.3) | 0.005 | 110 | 56 (76.7) | 54 (74.0) | 0.848 |
| Female | 153 | 17 (23.3) | 136 (40.7) | 36 | 17 (23.3) | 19 (26.0) | ||
| Age (yr) | ||||||||
| ≤ 65 | 245 | 28 (38.4) | 217 (65.0) | < 0.001 | 62 | 28 (38.4) | 34 (46.6) | 0.403 |
| > 65 | 162 | 45 (61.6) | 117 (35.0) | 84 | 45 (61.6) | 39 (53.4) | ||
| Binet stage | ||||||||
| A | 204 | 28 (38.4) | 176 (52.7) | 0.028 | 60 | 28 (38.4) | 32 (43.8) | 0.614 |
| B/C | 203 | 45 (61.6) | 158 (47.3) | 86 | 45 (61.6) | 41 (56.2) | ||
| ECOG PS | ||||||||
| 0-1 | 351 | 57 (78.1) | 294 (88.0) | 0.037 | 116 | 57 (78.1) | 59 (80.8) | 0.838 |
| > 1 | 56 | 16 (21.9) | 40 (12.0) | 30 | 16 (21.9) | 14 (19.2) | ||
| Richter transformation | ||||||||
| Presence | 10 | 5 (6.8) | 5 (1.5) | 0.020 | 10 | 5 (6.8) | 5 (6.8) | 1.000 |
| Absence | 397 | 68 (93.2) | 329 (98.5) | 136 | 68 (93.2) | 68 (93.2) | ||
| CLL-IPI | ||||||||
| 0-3 | 266 | 29 (39.7) | 237 (71.0) | < 0.001 | 63 | 29 (39.7) | 34 (46.6) | 0.504 |
| 4-10 | 141 | 44 (60.3) | 97 (29.0) | 83 | 44 (60.3) | 39 (53.4) | ||
| ALC (×109/L) | ||||||||
| ≤ 50 | 325 | 54 (74.0) | 271 (81.1) | 0.197 | 112 | 54 (74.0) | 58 (79.5) | 0.557 |
| > 50 | 82 | 19 (26.0) | 63 (18.9) | 34 | 19 (26.0) | 15 (20.5) | ||
| Hb (g/L) | ||||||||
| < 100 | 54 | 21 (28.8) | 33 (9.9) | < 0.001 | 36 | 21 (28.8) | 15 (20.5) | 0.337 |
| ≥ 100 | 353 | 52 (71.2) | 301 (90.1) | 110 | 52 (71.2) | 58 (79.5) | ||
| PLT (×109/L) | ||||||||
| < 100 | 65 | 25 (34.3) | 40 (12.0) | < 0.001 | 44 | 25 (34.3) | 19 (26.0) | 0.367 |
| ≥ 100 | 342 | 48 (65.7) | 294 (88.0) | 102 | 48 (65.7) | 54 (74.0) | ||
| LDH (271 U/L) | ||||||||
| ≤ ULN | 333 | 55 (75.3) | 278 (83.2) | 0.131 | 107 | 55 (75.3) | 52 (71.2) | 0.709 |
| > ULN | 74 | 18 (24.7) | 56 (16.8) | 39 | 18 (24.7) | 21 (28.8) | ||
| Albumin (3.5 g/dL) | ||||||||
| < LLN | 132 | 28 (38.4) | 104 (31.1) | 0.270 | 56 | 28 (38.4) | 28 (38.4) | 1.000 |
| ≥ LLN | 275 | 45 (61.6) | 230 (68.9) | 90 | 45 (61.6) | 45 (61.6) | ||
| β2-MG (g/L) | ||||||||
| ≤ 3.50 | 244 | 30 (41.1) | 214 (64.1) | < 0.001 | 61 | 30 (41.1) | 31 (42.5) | 1.000 |
| > 3.50 | 163 | 43 (58.9) | 120 (35.9) | 85 | 43 (58.9) | 42 (57.5) | ||
| CRP (1 mg/dL) | ||||||||
| ≤ ULN | 331 | 56 (76.7) | 275 (82.3) | 0.319 | 114 | 56 (76.7) | 58 (79.5) | 0.842 |
| > ULN | 76 | 17 (23.3) | 59 (17.7) | 32 | 17 (23.3) | 15 (20.5) | ||
| Treatments | ||||||||
| Intensive treatmentsc) | 155 | 40 (54.8) | 115 (34.4) | 0.174 | 73 | 40 (54.8) | 33 (45.2) | 0.377 |
| Less intensive treatmentsd) | 71 | 12 (16.4) | 59 (17.7) | 27 | 12 (16.4) | 15 (20.6) | ||
| Biological variable | ||||||||
| TP53 disruption | ||||||||
| Presence | 65 | 22 (30.1) | 43 (12.9) | 0.001 | 39 | 22 (30.1) | 17 (23.3) | 0.455 |
| Absence | 342 | 51 (69.9) | 291 (87.1) | 107 | 51 (69.9) | 56 (76.7) | ||
| ATM deletion | ||||||||
| Presence | 47 | 10 (13.7) | 37 (11.1) | 0.545 | 17 | 10 (13.7) | 7 (9.6) | 0.607 |
| Absence | 360 | 63 (86.3) | 297 (88.9) | 129 | 63 (86.3) | 66 (90.4) | ||
| IGHV | ||||||||
| Unmutated | 137 | 33 (45.2) | 104 (31.1) | 0.028 | 65 | 33 (45.2) | 32 (43.8) | 1.000 |
| Mutated | 270 | 40 (54.8) | 230 (68.9) | 81 | 40 (54.8) | 41 (56.2) | ||
| CD38 (%) | ||||||||
| < 30 | 320 | 56 (76.7) | 264 (79.0) | 0.639 | 115 | 56 (76.7) | 59 (80.8) | 0.686 |
| ≥ 30 | 87 | 17 (23.3) | 70 (21.0) | 31 | 17 (23.3) | 14 (19.2) | ||
| ZAP-70 (%) | ||||||||
| < 20 | 241 | 41 (56.2) | 200 (59.9) | 0.600 | 86 | 41 (56.2) | 45 (61.6) | 0.614 |
| ≥ 20 | 166 | 32 (43.8) | 134 (40.1) | 60 | 32 (43.8) | 28 (38.4) | ||
Values are presented as number (%). CLL, chronic lymphocytic leukemia; TTFT, time-to-first-treatment; ECOG, Eastern Cooperative Oncology Group; PS, performance status; IPI, international prognostic index; ALC, absolute lymphocytic count; Hb, hemoglobin; PLT, platelet; LDH, lactate dehydrogenase; ULN, upper limit of normal; LLN, lower limit of normal; β2-MG, β2-microglobulin; CRP, C-reactive protein; IGHV, immu-noglobulin heavy chain variable region; ZAP-70, zeta-chain associated protein kinase 70.
Table 2.
| Variable |
Unmatched (complete) dataset |
Propensity score-matched (1:1) datasetb) |
||||||
|---|---|---|---|---|---|---|---|---|
| Total | Diabetic (n=111) | Non-diabetic (n=522) | p-value | Total | Diabetic (n=111) | Non-diabetic (n=111) | p-value | |
| Clinical variable | ||||||||
| Sex | ||||||||
| Male | 409 | 82 (73.9) | 327 (62.6) | 0.029 | 156 | 82 (73.9) | 74 (66.7) | 0.304 |
| Female | 224 | 29 (26.1) | 195 (37.4) | 66 | 29 (26.1) | 37 (33.3) | ||
| Age (yr) | ||||||||
| ≤ 65 | 394 | 57 (51.4) | 337 (64.6) | 0.013 | 120 | 57 (51.4) | 63 (56.8) | 0.501 |
| > 65 | 239 | 54 (48.6) | 185 (35.4) | 102 | 54 (48.6) | 48 (43.2) | ||
| Binet stage | ||||||||
| A | 232 | 33 (29.7) | 199 (38.1) | 0.104 | 65 | 33 (29.7) | 32 (28.8) | 1.000 |
| B/C | 401 | 78 (70.3) | 323 (61.9) | 157 | 78 (70.3) | 79 (71.2) | ||
| ECOG PS | ||||||||
| 0-1 | 529 | 85 (76.6) | 444 (85.1) | 0.034 | 176 | 85 (76.6) | 91 (82.0) | 0.408 |
| > 1 | 104 | 26 (23.4) | 78 (14.9) | 46 | 26 (23.4) | 20 (18.0) | ||
| Richter transformation | ||||||||
| Presence | 29 | 9 (8.1) | 20 (3.8) | 0.075 | 13 | 9 (8.1) | 4 (3.6) | 0.252 |
| Absence | 604 | 102 (91.9) | 502 (96.2) | 209 | 102 (91.9) | 107 (96.4) | ||
| CLL-IPI | ||||||||
| 0-3 | 361 | 42 (37.8) | 319 (61.1) | < 0.001 | 90 | 42 (37.8) | 48 (43.2) | 0.494 |
| 4-10 | 272 | 69 (62.2) | 203 (38.9) | 132 | 69 (62.2) | 63 (56.8) | ||
| ALC (×109/L) | ||||||||
| ≤ 50 | 485 | 83 (74.8) | 402 (77.0) | 0.622 | 159 | 83 (74.8) | 76 (68.5) | 0.372 |
| > 50 | 148 | 28 (25.2) | 120 (23.0) | 63 | 28 (25.2) | 35 (31.5) | ||
| Hb (g/L) | ||||||||
| < 100 | 138 | 41 (36.9) | 97 (18.6) | < 0.001 | 73 | 41 (36.9) | 32 (28.8) | 0.253 |
| ≥ 100 | 495 | 70 (63.1) | 425 (81.4) | 149 | 70 (63.1) | 79 (71.2) | ||
| PLT (×109/L) | ||||||||
| < 100 | 183 | 50 (45.1) | 133 (25.5) | < 0.001 | 88 | 50 (45.1) | 38 (34.2) | 0.131 |
| ≥ 100 | 450 | 61 (54.9) | 389 (74.5) | 134 | 61 (54.9) | 73 (65.8) | ||
| LDH (271 U/L) | ||||||||
| ≤ ULN | 487 | 74 (66.7) | 413 (79.1) | 0.006 | 153 | 74 (66.7) | 79 (71.2) | 0.562 |
| > ULN | 146 | 37 (33.3) | 109 (20.9) | 69 | 37 (33.3) | 32 (28.8) | ||
| Albumin (3.5 g/dL) | ||||||||
| < LLN | 243 | 51 (45.9) | 192 (36.8) | 0.085 | 96 | 51 (45.9) | 45 (40.5) | 0.498 |
| ≥ LLN | 390 | 60 (54.1) | 330 (63.2) | 126 | 60 (54.1) | 66 (59.5) | ||
| β2-MG (mg/L) | ||||||||
| ≤ 3.50 | 345 | 48 (43.2) | 297 (56.9) | 0.012 | 100 | 48 (43.2) | 52 (46.8) | 0.686 |
| > 3.50 | 288 | 63 (56.8) | 225 (43.1) | 122 | 63 (56.8) | 59 (53.2) | ||
| CRP (1 mg/dL) | ||||||||
| ≤ ULN | 497 | 83 (74.8) | 414 (79.3) | 0.309 | 165 | 83 (74.8) | 82 (73.9) | 1.000 |
| > ULN | 136 | 28 (25.2) | 108 (20.7) | 57 | 28 (25.2) | 29 (26.1) | ||
| Treatments | ||||||||
| Intensive treatmentsc) | 322 | 71 (64.0) | 251 (48.1) | 0.090 | 135 | 71 (64.0) | 64 (57.7) | 0.302 |
| Less intensive treatmentsd) | 130 | 19 (17.1) | 111 (21.3) | 44 | 19 (17.1) | 25 (22.5) | ||
| Biological variable | ||||||||
| TP53 disruption | ||||||||
| Presence | 132 | 40 (36.0) | 92 (17.6) | < 0.001 | 72 | 40 (36.0) | 32 (28.8) | 0.316 |
| Absence | 501 | 71 (64.0) | 430 (82.4) | 150 | 71 (64.0) | 79 (71.2) | ||
| ATM deletion | ||||||||
| Presence | 77 | 15 (13.5) | 62 (11.9) | 0.632 | 32 | 15 (13.5) | 17 (15.3) | 0.849 |
| Absence | 556 | 96 (86.5) | 460 (88.1) | 190 | 96 (86.5) | 94 (84.7) | ||
| IGHV | ||||||||
| Unmutated | 253 | 53 (47.7) | 200 (38.3) | 0.070 | 105 | 53 (47.7) | 52 (46.8) | 1.000 |
| Mutated | 380 | 58 (52.3) | 322 (61.7) | 117 | 58 (52.3) | 59 (53.2) | ||
| CD38 (%) | ||||||||
| < 30 | 473 | 81 (73.0) | 392 (75.1) | 0.632 | 161 | 81 (73.0) | 80 (72.1) | 1.000 |
| ≥ 30 | 160 | 30 (27.0) | 130 (24.9) | 61 | 30 (27.0) | 31 (27.9) | ||
| ZAP-70 (%) | ||||||||
| < 20 | 383 | 64 (57.7) | 319 (61.1) | 0.522 | 127 | 64 (57.7) | 63 (56.8) | 1.000 |
| ≥ 20 | 250 | 47 (42.3) | 203 (38.9) | 95 | 47 (42.3) | 48 (43.2) | ||
Values are presented as number (%). CLL, chronic lymphocytic leukemia; CSS, cancer-specific survival; ECOG, Eastern Cooperative Oncology Group; PS, performance status; IPI, international prognostic index; ALC, absolute lymphocytic count; Hb, hemoglobin; PLT, platelet; LDH, lactate dehydrogenase; ULN, upper limit of normal; LLN, lower limit of normal; β2-MG, β2-microglobulin; CRP, C-reactive protein; IGHV, immu-noglobulin heavy chain variable region; ZAP-70, zeta-chain associated protein kinase 70.
2. Data collection
3. Definition of pre-existing DM
4. Follow-up and outcome measures
5. Statistical analyses
6. Ethical statement
Results
1. Patients’ clinical characteristics in relation to DM
2. Prognostic value of pre-existing DM in CLL
Fig. 1.
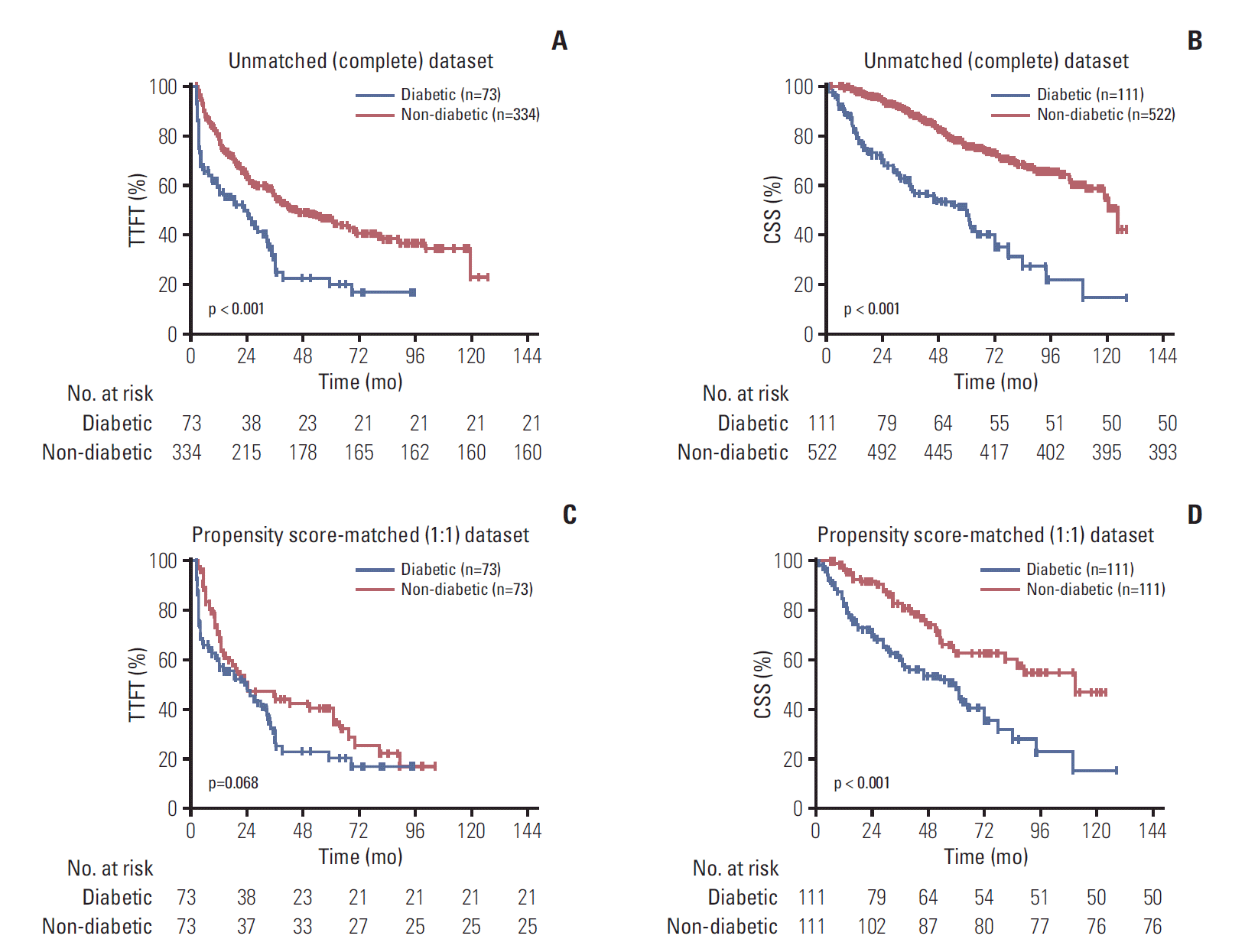
Table 3.
| Variable |
Unmatched (complete) dataset |
Propensity score-matched (1:1) dataseta) |
||||||
|---|---|---|---|---|---|---|---|---|
|
Univariate analyses |
Multivariate analyses |
Univariate analyses |
Multivariate analyses |
|||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| TTFT | ||||||||
| Male | 1.127 (0.859-1.478) | 0.390 | 0.980 (0.731-1.314) | 0.893 | 1.022 (0.649-1.608) | 0.925 | 0.964 (0.588-1.581) | 0.886 |
| Age | 1.205 (0.926-1.568) | 0.166 | 0.836 (0.619-1.129) | 0.243 | 1.129 (0.759-1.680) | 0.550 | 0.907 (0.576-1.428) | 0.674 |
| Binet B/C | 2.701 (2.055-3.549) | < 0.001 | 1.901 (1.366-2.644) | < 0.001 | 3.917 (2.484-6.177) | < 0.001 | 3.296 (1.865-5.828) | < 0.001 |
| ECOG PS > 1 | 1.444 (1.018-2.047) | 0.039 | 1.523 (1.027-2.257) | 0.036 | 1.210 (0.758-1.930) | 0.425 | 1.773 (1.043-3.013) | 0.034 |
| Hb < 100 g/L | 2.372 (1.708-3.293) | < 0.001 | 0.883 (0.597-1.308) | 0.536 | 2.417 (1.587-3.682) | < 0.001 | 0.896 (0.535-1.501) | 0.676 |
| PLT < 100×109/L | 3.030 (2.224-4.127) | < 0.001 | 1.577 (1.082-2.301) | 0.018 | 2.371 (1.576-3.566) | < 0.001 | 0.977 (0.582-1.639) | 0.930 |
| LDH > ULN (271 U/L) | 1.966 (1.455-2.655) | < 0.001 | 1.293 (0.908-1.839) | 0.154 | 2.225 (1.461-3.387) | < 0.001 | 1.452 (0.857-2.461) | 0.166 |
| β2-MG > 3.50 mg/L | 2.118 (1.630-2.751) | < 0.001 | 1.378 (1.022-1.857) | 0.036 | 2.224 (1.448-3.416) | < 0.001 | 1.518 (0.938-2.456) | 0.089 |
| TP53 disruption | 2.504 (1.816-3.453) | < 0.001 | 1.577 (1.114-2.234) | 0.010 | 2.898 (1.861-4.513) | < 0.001 | 1.933 (1.169-3.196) | 0.010 |
| ATM deletion | 1.444 (0.988-2.111) | 0.058 | 1.104 (0.747-1.633) | 0.619 | 1.295 (0.720-2.329) | 0.388 | 0.972 (0.519-1.820) | 0.929 |
| IGHV unmutated | 2.493 (1.910-3.255) | < 0.001 | 1.919 (1.425-2.584) | < 0.001 | 2.424 (1.622-3.621) | < 0.001 | 1.655 (0.991-2.765) | 0.054 |
| CD38 ≥ 30% | 1.320 (0.969-1.797) | 0.078 | 1.129 (0.803-1.586) | 0.485 | 1.205 (0.748-1.940) | 0.443 | 1.163 (0.664-2.038) | 0.597 |
| ZAP-70 ≥ 20% | 1.043 (0.797-1.365) | 0.761 | 0.863 (0.648-1.148) | 0.312 | 1.111 (0.741-1.665) | 0.611 | 1.007 (0.639-1.588) | 0.975 |
| Diabetic | 2.046 (1.498-2.794) | < 0.001 | 1.639 (1.170-2.297) | 0.004 | 1.444 (0.973-2.144) | 0.068 | 1.580 (1.041-2.398) | 0.032 |
| CSS | ||||||||
| Male | 1.294 (0.953-1.756) | 0.098 | 1.118 (0.812-1.541) | 0.494 | 1.094 (0.704-1.701) | 0.689 | 1.026 (0.641-1.645) | 0.914 |
| Age | 1.878 (1.413-2.496) | < 0.001 | 1.876 (1.378-2.554) | < 0.001 | 1.320 (0.883-1.974) | 0.176 | 1.691 (1.069-2.678) | 0.025 |
| Binet B/C | 2.894 (2.032-4.123) | < 0.001 | 2.029 (1.319-3.122) | 0.001 | 2.021 (1.222-3.343) | 0.006 | 1.656 (0.872-3.147) | 0.123 |
| ECOG PS > 1 | 1.860 (1.331-2.600) | < 0.001 | 1.405 (0.985-2.002) | 0.060 | 1.215 (0.754-1.956) | 0.423 | 1.304 (0.784-2.168) | 0.306 |
| Hb < 100 g/L | 2.052 (1.519-2.771) | < 0.001 | 1.046 (0.731-1.498) | 0.805 | 1.495 (0.994-2.249) | 0.053 | 0.988 (0.611-1.597) | 0.959 |
| PLT < 100×109/L | 2.027 (1.522-2.701) | < 0.001 | 0.872 (0.612-1.243) | 0.449 | 1.538 (1.030-2.296) | 0.035 | 0.858 (0.528-1.394) | 0.536 |
| LDH > ULN (271 U/L) | 2.408 (1.798-3.224) | < 0.001 | 1.329 (0.942-1.875) | 0.105 | 2.043 (1.365-3.056) | 0.001 | 1.292 (0.781-2.137) | 0.319 |
| β2-MG > 3.50 mg/L | 2.395 (1.775-3.230) | < 0.001 | 1.523 (1.098-2.112) | 0.012 | 1.704 (1.109-2.618) | 0.015 | 1.363 (0.864-2.152) | 0.183 |
| TP53 disruption | 3.283 (2.444-4.410) | < 0.001 | 1.934 (1.388-2.695) | < 0.001 | 2.500 (1.663-3.757) | < 0.001 | 1.975 (1.240-3.144) | 0.004 |
| ATM deletion | 1.435 (0.966-2.132) | 0.074 | 1.136 (0.758-1.702) | 0.537 | 1.280 (0.737-2.223) | 0.381 | 1.372 (0.778-2.419) | 0.274 |
| IGHV unmutated | 2.947 (2.199-3.950) | < 0.001 | 1.960 (1.424-2.697) | < 0.001 | 2.229 (1.466-3.389) | < 0.001 | 1.730 (1.088-2.751) | 0.020 |
| CD38 ≥ 30% | 1.174 (0.854-1.615) | 0.323 | 0.882 (0.634-1.225) | 0.453 | 1.067 (0.686-1.660) | 0.772 | 0.933 (0.590-1.474) | 0.765 |
| ZAP-70 ≥ 20% | 0.991 (0.736-1.337) | 0.955 | 0.752 (0.553-1.023) | 0.070 | 0.958 (0.636-1.442) | 0.836 | 0.816 (0.535-1.244) | 0.344 |
| Diabetic | 3.469 (2.549-4.722) | < 0.001 | 2.758 (1.989-3.824) | <0.001 | 2.332 (1.532-3.549) | < 0.001 | 2.514 (1.613-3.918) | < 0.001 |
TTFT, time-to-first-treatment; CSS, cancer-specific survival; HR, hazard ratio; 95% CI, 95% confidence interval; ECOG, Eastern Cooperative Oncology Group; PS, performance status; Hb, hemoglobin; PLT, platelet; LDH, lactate dehydrogenase; ULN, upper limit of normal; β2-MG, β2-microglobulin; IGHV, immunoglobulin heavy chain variable region; ZAP-70, zeta-chain associated protein kinase 70.
3. Analyses of prediabetes, diabetic duration and HbA1c in relation to CLL prognosis
Fig. 2.
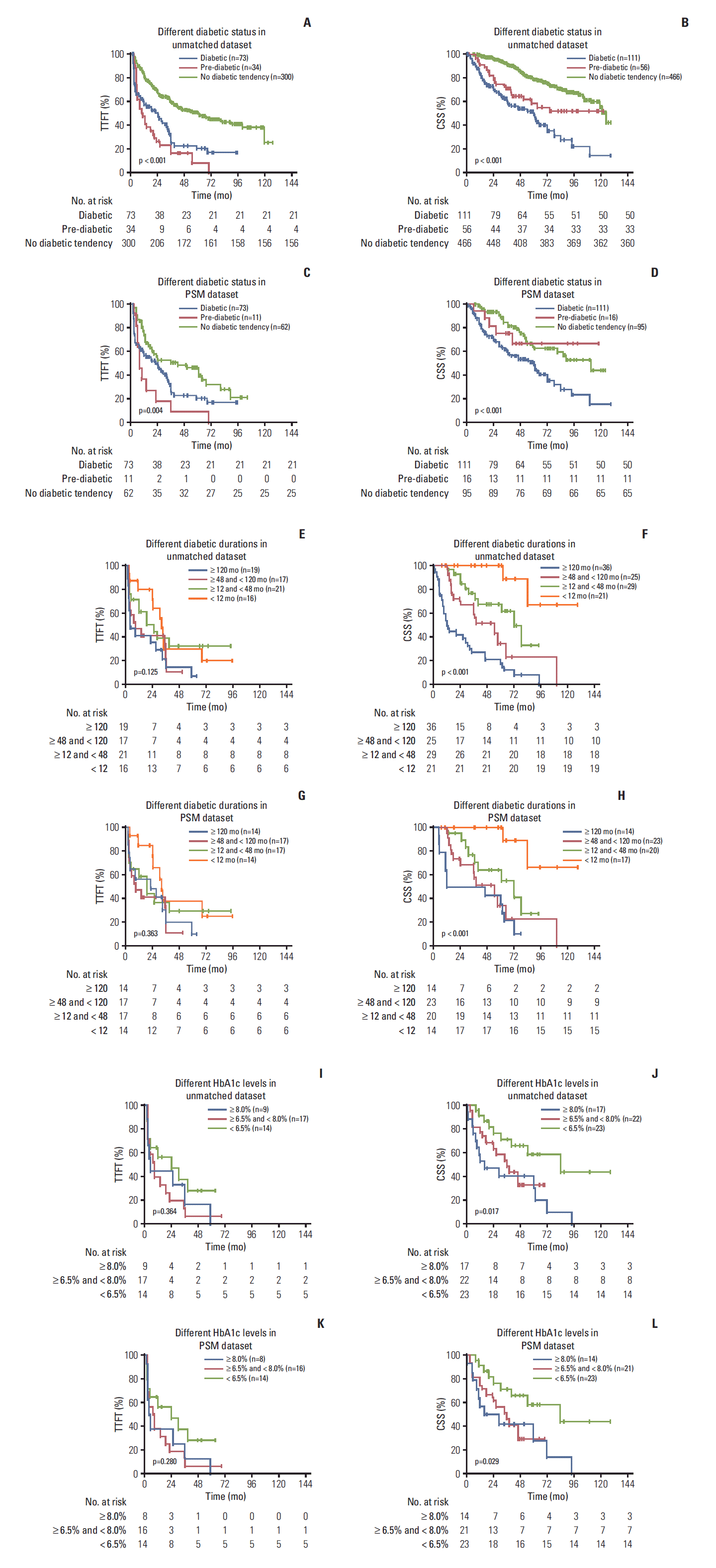
4. DM together with CLL-IPI: a better prognostic index for CLL
Fig. 3.
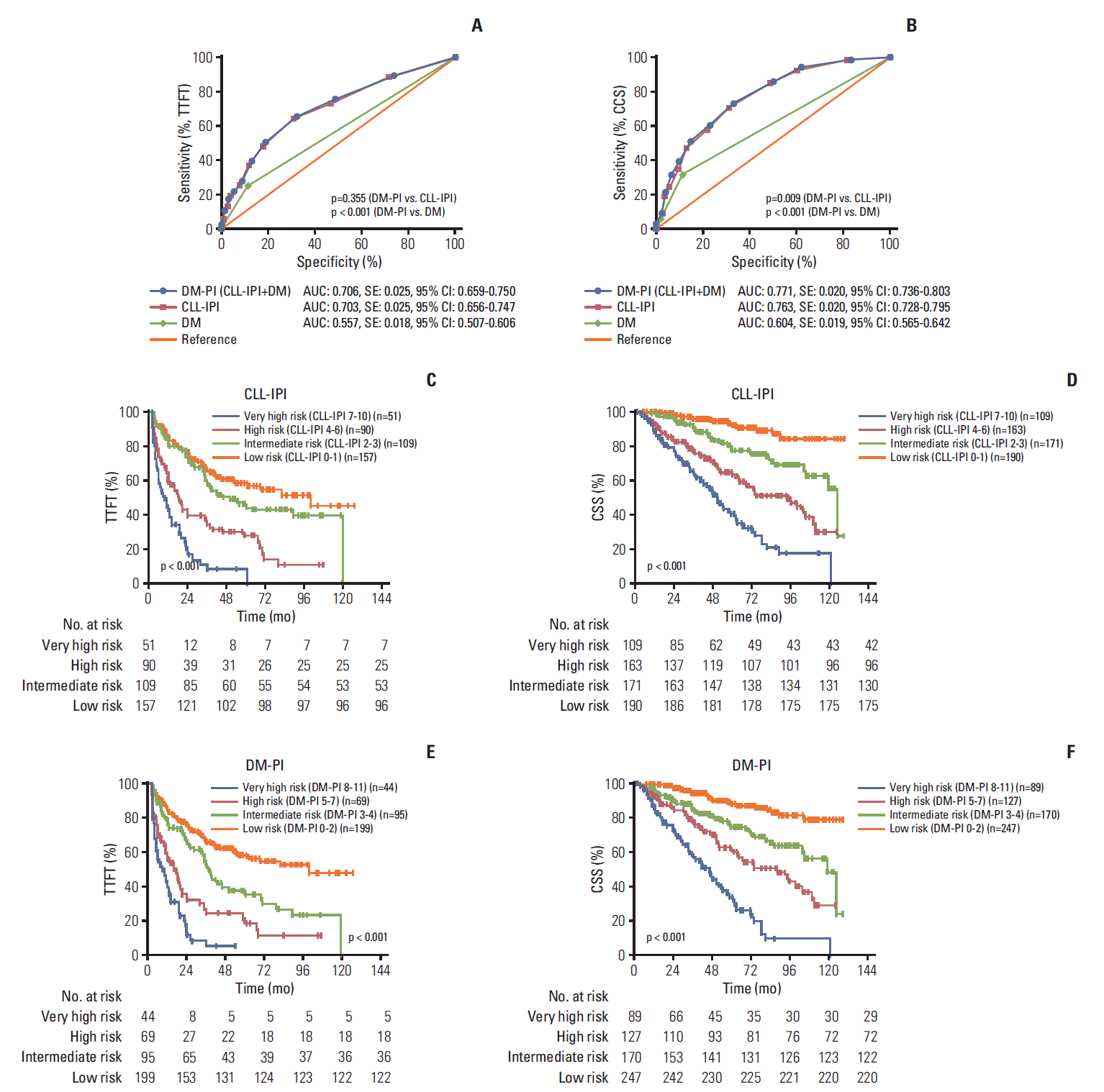




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download