1. Neale G, Woloshynowych M, Vincent C. Exploring the causes of adverse events in NHS hospital practice. J R Soc Med. 2001; 94:322–30.

2. Jones DA, DeVita MA, Bellomo R. Rapid-response teams. N Engl J Med. 2011; 365:139–46.

3. Devita MA, Bellomo R, Hillman K, Kellum J, Rotondi A, Teres D, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006; 34:2463–78.

4. Hillman K, Chen J, Cretikos M, Bellomo R, Brown D, Doig G, et al. Introduction of the medical emergency team (MET) system: a cluster-randomised controlled trial. Lancet. 2005; 365:2091–7.
5. Chan PS, Jain R, Nallmothu BK, Berg RA, Sasson C. Rapid response teams: a systematic review and meta-analysis. Arch Intern Med. 2010; 170:18–26.
6. Jones D, Bellomo R, DeVita MA. Effectiveness of the Medical Emergency Team: the importance of dose. Crit Care. 2009; 13:313.

7. Bellomo R, Goldsmith D, Uchino S, Buckmaster J, Hart GK, Opdam H, et al. A prospective before-and-after trial of a medical emergency team. Med J Aust. 2003; 179:283–7.

8. Sebat F, Musthafa AA, Johnson D, Kramer AA, Shoffner D, Eliason M, et al. Effect of a rapid response system for patients in shock on time to treatment and mortality during 5 years. Crit Care Med. 2007; 35:2568–75.

9. Buist M, Harrison J, Abaloz E, Van Dyke S. Six year audit of cardiac arrests and medical emergency team calls in an Australian outer metropolitan teaching hospital. BMJ. 2007; 335:1210–2.

10. Jung B, Daurat A, De Jong A, Chanques G, Mahul M, Monnin M, et al. Rapid response team and hospital mortality in hospitalized patients. Intensive Care Med. 2016; 42:494–504.

11. Salvatierra G, Bindler RC, Corbett C, Roll J, Daratha KB. Rapid response team implementation and in-hospital mortality. Crit Care Med. 2014; 42:2001–6.

12. Wilson RM, Runciman WB, Gibberd RW, Harrison BT, Newby L, Hamilton JD. The quality in Australian Health Care Study. Med J Aust. 1995; 163:458–71.

13. Schwendimann R, Blatter C, Dhaini S, Simon M, Ausserhofer D. The occurrence, types, consequences and preventability of in-hospital adverse events: a scoping review. BMC Health Serv Res. 2018; 18:521.

14. Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990; 98:1388–92.

15. Buist M, Bernard S, Nguyen TV, Moore G, Anderson J. Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation. 2004; 62:137–41.

16. Andersen LW, Kim WY, Chase M, Berg KM, Mortensen SJ, Moskowitz A, et al. The prevalence and significance of abnormal vital signs prior to in-hospital cardiac arrest. Resuscitation. 2016; 98:112–7.

17. Smith GB, Prytherch DR, Schmidt P, Featherstone PI, Knight D, Clements G, et al. Hospital-wide physiological surveillancea new approach to the early identification and management of the sick patient. Resuscitation. 2006; 71:19–28.

18. Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Crit Care Med. 2018; 46:997–1000.

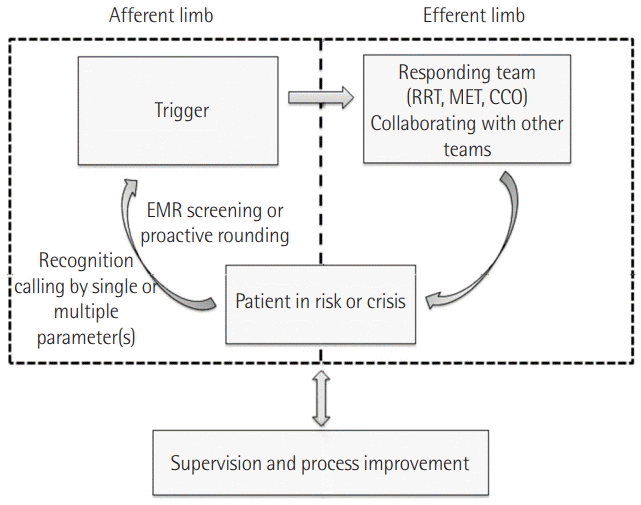
19. Taenzer AH, Spence BC. The afferent limb of rapid response systems: continuous monitoring on general care units. Crit Care Clin. 2018; 34:189–98.
20. Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified Early Warning Score in medical admissions. QJM. 2001; 94:521–6.

21. McGaughey J, Alderdice F, Fowler R, Kapila A, Mayhew A, Moutray M. Outreach and Early Warning Systems (EWS) for the prevention of intensive care admission and death of critically ill adult patients on general hospital wards. Cochrane Database Syst Rev. 2007; (3):CD005529.

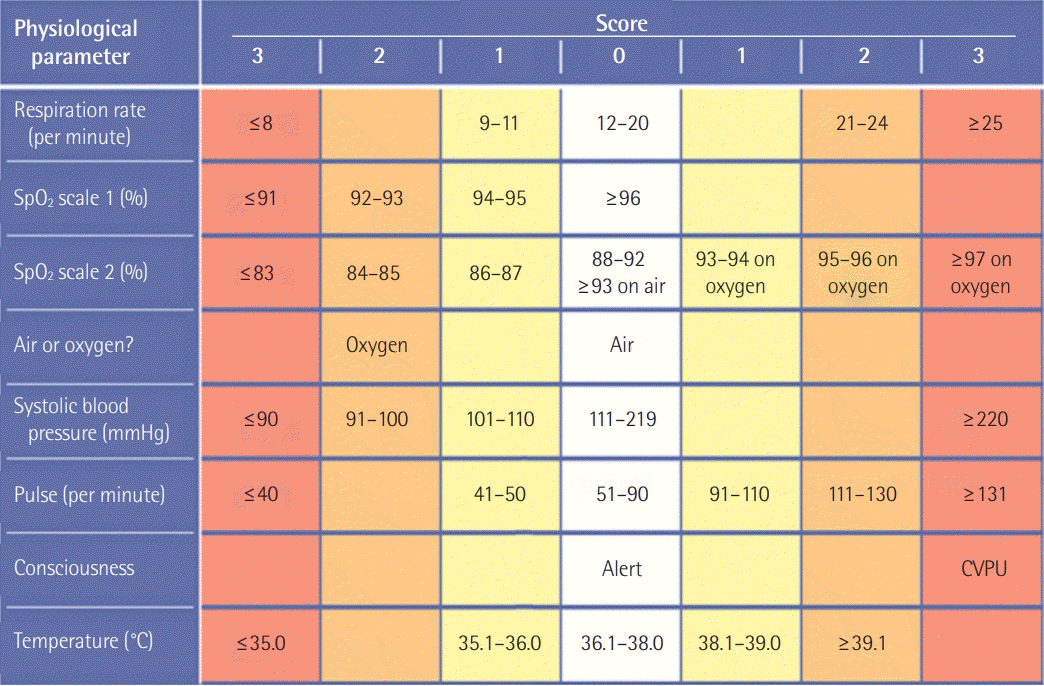
22. Royal College of Physicians. National Early Warning Score (NEWS) 2: standardising the assessment of acute-illness severity in the NHS. London: Royal College of Physicians;2017.
23. Berwick DM, Calkins DR, McCannon CJ, Hackbarth AD. The 100,000 lives campaign: setting a goal and a deadline for improving health care quality. JAMA. 2006; 295:324–7.
24. The Joint Commission announces the 2009 National Patient Safety Goals and requirements. Jt Comm Perspect. 2008; 28:111–5.
25. Kronick SL, Kurz MC, Lin S, Edelson DP, Berg RA, Billi JE, et al. Part 4: systems of care and continuous quality improvement. 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015; 132(18 Suppl 2):S397–413.

26. Konrad D, Jäderling G, Bell M, Granath F, Ekbom A, Martling CR. Reducing in-hospital cardiac arrests and hospital mortality by introducing a medical emergency team. Intensive Care Med. 2010; 36:100–6.

27. Chan PS, Khalid A, Longmore LS, Berg RA, Kosiborod M, Spertus JA. Hospital-wide code rates and mortality before and after implementation of a rapid response team. JAMA. 2008; 300:2506–13.

28. Edelson DP, Yuen TC, Mancini ME, Davis DP, Hunt EA, Miller JA, et al. Hospital cardiac arrest resuscitation practice in the United States: a nationally representative survey. J Hosp Med. 2014; 9:353–7.

29. Priestley G, Watson W, Rashidian A, Mozley C, Russell D, Wilson J, et al. Introducing Critical Care Outreach: a ward-randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004; 30:1398–404.

30. Jones D, Bellomo R, Bates S, Warrillow S, Goldsmith D, Hart G, et al. Long term effect of a medical emergency team on cardiac arrests in a teaching hospital. Crit Care. 2005; 9:R808–15.
31. Buist MD, Moore GE, Bernard SA, Waxman BP, Anderson JN, Nguyen TV. Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002; 324:387–90.

32. Bellomo R, Goldsmith D, Uchino S, Buckmaster J, Hart G, Opdam H, et al. Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates. Crit Care Med. 2004; 32:916–21.

33. Maharaj R, Raffaele I, Wendon J. Rapid response systems: a systematic review and meta-analysis. Crit Care. 2015; 19:254.

34. ANZICS-CORE MET dose investigators. Mortality of rapid response team patients in Australia: a multicentre study. Crit Care Resusc. 2013; 15:273–8.
35. Huh JW, Lim CM, Koh Y, Lee J, Jung YK, Seo HS, et al. Activation of a medical emergency team using an electronic medical recording-based screening system. Crit Care Med. 2014; 42:801–8.

36. Kwak HJ, Yun I, Kim SH, Sohn JW, Shin DH, Yoon HJ, et al. The extended rapid response system: 1-year experience in a university hospital. J Korean Med Sci. 2014; 29:423–30.

37. Lee YJ, Park JJ, Yoon YE, Kim JW, Park JS, Kim T, et al. Successful implementation of a rapid response system in the department of internal medicine. Korean J Crit Care Med. 2014; 29:77–82.

38. Kim Y, Lee DS, Min H, Choi YY, Lee EY, Song I, et al. Effectiveness analysis of a part-time rapid response system during operation versus nonoperation. Crit Care Med. 2017; 45:e592–9.

39. Park Y, Ahn JJ, Kang BJ, Lee YS, Ha SO, Min JS, et al. Rapid response systems reduce in-hospital cardiopulmonary arrest: a pilot study and motivation for a nationwide survey. Korean J Crit Care Med. 2017; 32:231–9.

40. Song JU, Suh GY, Park HY, Lim SY, Han SG, Kang YR, et al. Early intervention on the outcomes in critically ill cancer patients admitted to intensive care units. Intensive Care Med. 2012; 38:1505–13.

41. Lee SH, Leem AY, Nho Y, Kim YA, Kim KD, Kim YS, et al. A pilot study of the effectiveness of medical emergency system implementation at a single center in Korea. Korean J Crit Care Med. 2017; 32:133–41.

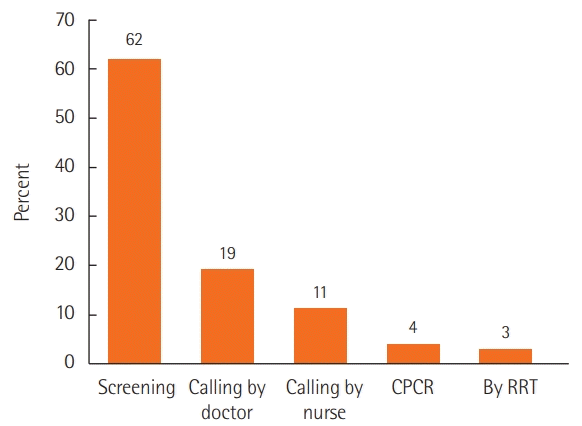
42. Kim SW, Lee HY, Han MR, Lee YS, Kang EH, Jang EJ, et al. Epidemiology and clinical characteristics of rapid response team activations. Korean J Crit Care Med. 2017; 32:124–32.

43. Lee DH, Han M, An JY, Jung JY, Koh Y, Lim CM, et al. Video laryngoscopy versus direct laryngoscopy for tracheal intubation during in-hospital cardiopulmonary resuscitation. Resuscitation. 2015; 89:195–9.

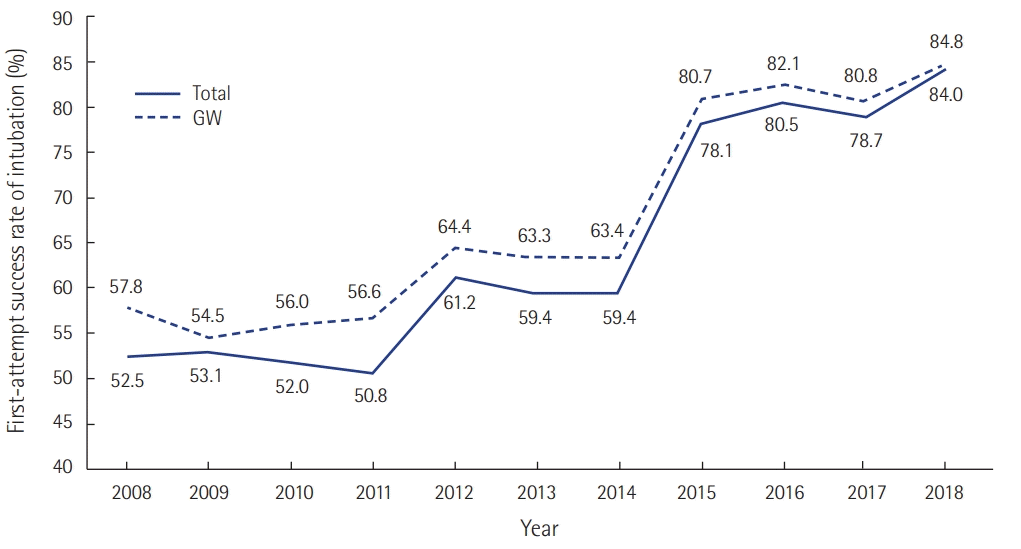
44. Baek MS, Han M, Huh JW, Lim CM, Koh Y, Hong SB. Video laryngoscopy versus direct laryngoscopy for first-attempt tracheal intubation in the general ward. Ann Intensive Care. 2018; 8:83.

45. Lyons PG, Edelson DP, Churpek MM. Rapid response systems. Resuscitation. 2018; 128:191–7.

46. Lee Y, Kwon JM, Lee Y, Park H, Cho H, Park J. Deep learning in the medical domain: predicting cardiac arrest using deep learning. Acute Crit Care. 2018; 33:117–20.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download