INTRODUCTION
MATERIALS AND METHODS
Study Design and Populations
Figure 1.
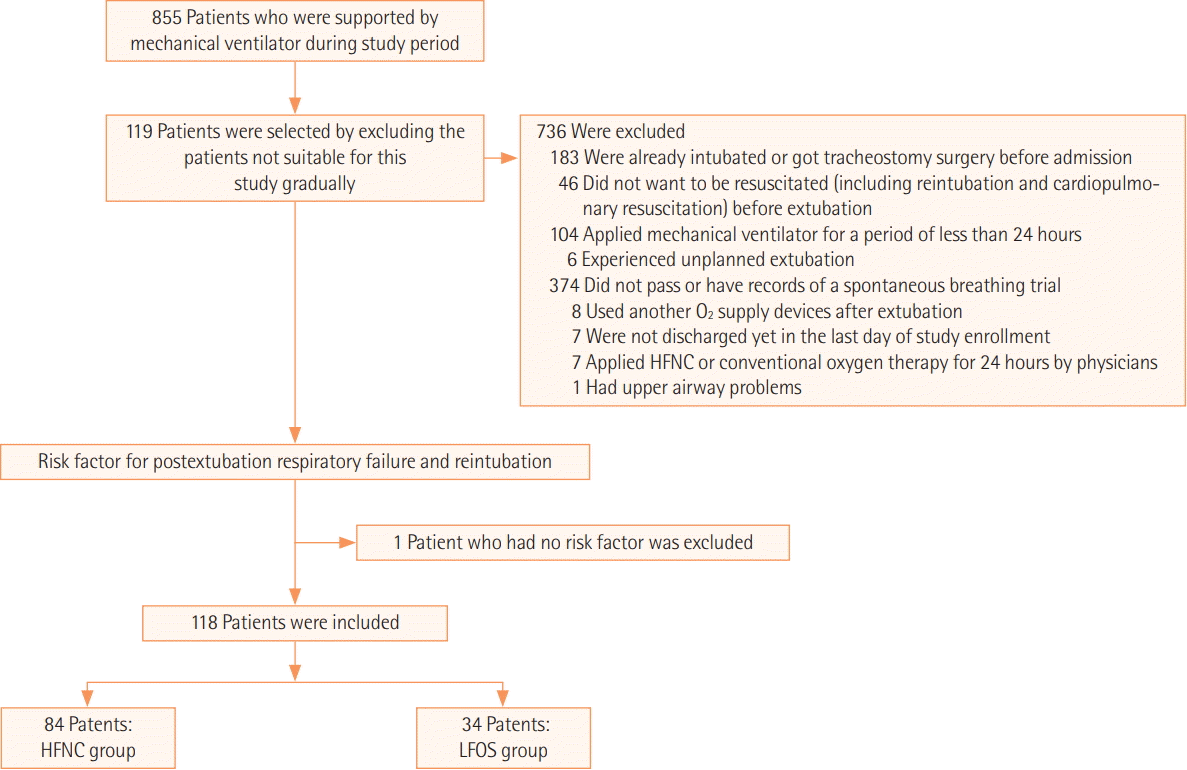
Data Collection
Statistical Analysis
RESULTS
Baseline Characteristics of the Enrolled Patients
Table 1.
| Characteristics | HFNC (n=84) | LFOS (n=34) | P-value |
|---|---|---|---|
| Male sex | 60 (71.4) | 20 (58.8) | 0.184 |
| Age (yr) | 73.0 (66.0–80.0) | 71.00 (55.75–81.25) | 0.454 |
| Height (cm) | 163.5 (158.0–170.0) | 155.50 (160.00–169.50) | 0.248 |
| Body weight (kg) | 56.65 (50.0–68.0) | 58.00 (52.00–65.00) | 0.983 |
| Body mass index (kg/m2) | 22.0 (18.8–24.5) | 23.11 (19.44–24.92) | 0.703 |
| Underlying disease | |||
| Diabetes mellitus | 19 (22.6) | 6 (17.6) | 0.549 |
| Hypertension | 31 (36.9) | 12 (35.3) | 0.869 |
| Malignant disease | 10 (11.9) | 4 (11.8) | 1.000a |
| Chronic respiratory disease | 47 (56.0) | 15 (44.1) | 0.244 |
| Chronic heart disease | 22 (26.2) | 7 (20.6) | 0.522a |
| Chronic liver disease | 1 (1.2) | 0 | 1.000a |
| Chronic renal disease | 11 (13.1) | 3 (8.8) | 0.755a |
| Neurologic disease | 27 (32.1) | 4 (11.8) | 0.023 |
| Cause of mechanical ventilation | |||
| Pneumonia | 45 (53.6) | 19 (55.9) | 0.819 |
| Airway disease | 13 (15.5) | 3 (8.8) | 0.119 |
| Hemoptysis | 3 (3.6) | 1 (2.9) | 1.000a |
| Drug intoxication | 14 (16.7) | 10 (29.4) | 0.119 |
| Post operation | 2 (2.4) | 0 | 1.000a |
| Heat failure | 3 (3.6) | 0 | 0.556a |
| Others | 4 (4.8) | 1 (2.9) | 1.000a |
| Type of respiratory failure at intubationb | |||
| Tachypneic respiratory failure | 6 (7.1) | 2 (5.9) | 1.000a |
| Hypercapnic respiratory failure | 38 (45.2) | 12 (35.3) | 0.322 |
| Hypoxic respiratory failure | 23 (27.4) | 6 (17.6) | 0.266a |
| Othersc | 22 (26.2) | 14 (41.2) | 0.109 |
| Severity index | |||
| APACHE II score at ICU admission | 22.0 (18.00–25.00) | 22.00 (19.00–25.25) | 0.466 |
| APACHE II score at extubation | 17.0 (14.0–19.0) | 16.50 (14.00–19.00) | 0.466 |
| Vital sign and arterial blood gas before intubation | |||
| Heart rate | 100.00 (85.00–120.00) | 107.50 (85.75–121.00) | 0.861 |
| Respiratory rate | 22.00 (18.00–27.50) | 22.00 (18.75–27.25) | 0.696 |
| PaCO2 (mmHg) | 41.75 (31.72–60.97) | 39.15 (32.15–53.55) | 0.671 |
| PaO2/FiO2 (mmHg) | 130.78 (83.76–259.29) | 255.71 (200.05–320.44) | 0.001 |
| (A–a) DO2 | 183.25 (50.71–412.68) | 56.12 (21.37–166.99) | 0.003 |
| Vital sign and arterial blood gas before extubation | |||
| Heart rate on ventilation | 84.50 (74.25–101.75) | 85.00 (68.00–90.25) | 0.198 |
| Respiratory rate on ventilation | 18.00 (16.00–21.00) | 17.00 (15.00–20.00) | 0.155 |
| PaCO2 on ventilation (mmHg) | 34.75 (30.05–40.35) | 34.40 (28.70–36.15) | 0.051 |
| PaO2/FiO2 on ventilation (mmHg) | 288.00 (208.31–363.81) | 333.75 (279.37–379.37) | 0.069 |
| (A-a) DO2 at spontaneous breathing trial | 138.71 (95.60–167.74) | 130.20 (88.29–130.20) | 0.478 |
| High risk patient | |||
| Age older than 65 years | 65 (77.4) | 20 (58.8) | 0.042 |
| Body mass index higher than 30 kg/m2 | 7 (8.3) | 2 (5.9) | 1.000a |
| Ventilator duration more than 7 days | 30 (35.7) | 7 (20.6) | 0.109 |
| Charlson comorbidity index of 2 or more | 33 (39.3) | 7 (20.6) | 0.052 |
| APACHE II score of more than 12 | 80 (95.2) | 32 (94.1) | 0.802 |
| Heart failure as a cause of intubation | 3 (3.6) | 0 | 0.556a |
| Moderate to severe COPD | 16 (19.0) | 0 | 0.005a |
| Failure with first SBT trial | 52 (61.9) | 17 (50.0) | 0.235 |
| Duration of mechanical ventilation before extubation (hr) | 120.3 (74.9–213.8) | 81.93 (47.45–139.09) | 0.012 |
| Hospital day before extubation trial (day) | 7.5 (4.00–10.75) | 4.5 (3.00–7.25) | 0.005 |
Values are presented as number (%) or median (interquartile range).
HFNC: high-flow nasal cannula; LFOS: low-flow oxygen system; APACHE: Acute Physiologic and Chronic Health Evaluation; ICU: intensive care unit; PaCO2: arterial partial pressure of carbon dioxide; PaO2/FiO2: ratio of arterial oxygen partial pressure to fractional inspired oxygen; (A–a) DO2: alveolar–arterial oxygen difference; COPD: chronic obstructive pulmonary disease; SBT: spontaneous breathing trial.
Results of Extubation: PERF and Reintubation
Table 2.
| Variable | HFNC (n=84) | LFOS (n=34) | χ2 | P-value |
|---|---|---|---|---|
| Reintubation | ||||
| Early reintubation (in 72 hr) | 8 (9.5) | 3 (8.8) | - | 1.000a |
| Time to reintubation | 10.41 (1.51–62.37) | 5.00 (4.17–43.85) | 0.838b | |
| Reintubation in 168 hr | 15 (17.9) | 3 (8.8) | 1.528 | 0.216 |
| Time to reintubation | 69.00 (2.58–100.82) | 5.00 (4.17–43.85) | 0.260b | |
| Delayed reintubation (72–168 hr) | 7/76c (9.2) | 0/31c | - | 0.105a |
| Postextubation respiratory failure | ||||
| Hypoxia | 7 (8.3) | 4 (11.8) | - | 0.727a |
| Hypercapnia | 2 (2.4) | 3 (8.8) | - | 0.143a |
| Tachypnea | 14 (16.7) | 6 (17.6) | 0.017 | 0.898 |
| All types of respiratory failure | 21 (25.0) | 11 (32.4) | 0.662 | 0.416 |
| Clinical outcome | ||||
| Tracheostomy | 5 (6.0) | 1 (2.9) | - | 0.672a |
| In hospital mortality | 12 (14.3) | 3 (8.8) | - | 0.549a |
| Hospital day | 27.5 (16.0–49.7) | 14.50 (9.0–31.0) | - | 0.001b |
Physiologic Effects of HFNC after Extubation
Figure 2.
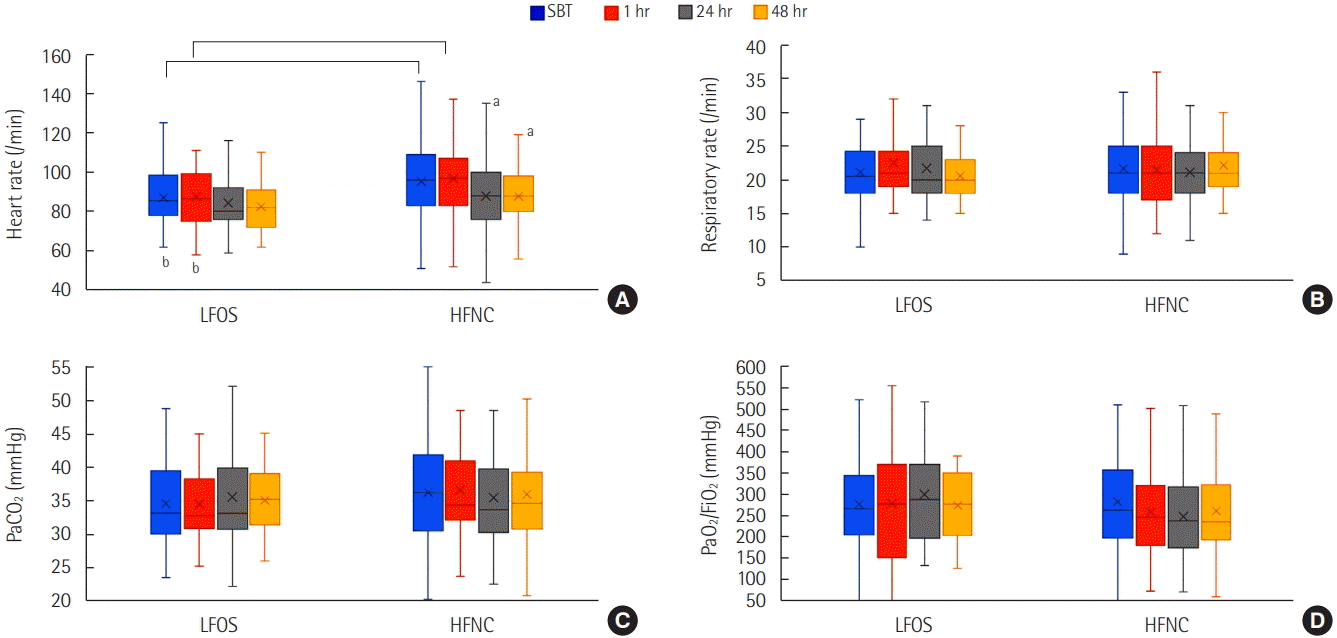
Predictors for Reintubation and Delayed Reintubation in the HFNC Group
Table 3.
| Characteristics | Non-reintubation (n=69) | Reintubation (n=15) | P-value |
|---|---|---|---|
| Male sex | 49 (71.0) | 11 (73.3) | 1.000a |
| Age (yr) | 74.0 (65.50–80.50) | 72.00 (67.00–74.00) | 0.245 |
| Height (cm) | 162.0 (158.0–170.0) | 164.50 (160.00–165.00) | 0.711 |
| Body weight (kg) | 58.0 (50.0–70.0) | 55.30 (50.00–60.00) | 0.656 |
| Body mass index (kg/m2) | 22.7 (18.7–24.9) | 22.03 (19.59–23.44) | 0.717 |
| Underlying disease | |||
| Diabetes mellitus | 15 (21.7) | 4 (26.7) | 0.736a |
| Hypertension | 25 (36.2) | 6 (40.0) | 0.784 |
| Malignant disease | 6 (8.7) | 4 (26.7) | 0.073a |
| Chronic respiratory disease | 40 (58.0) | 7 (46.7) | 0.424 |
| Chronic heart disease | 18 (26.1) | 4 (26.7) | 1.000a |
| Chronic liver disease | 1 (1.4) | 0 | 1.000a |
| Chronic renal disease | 7 (10.1) | 4 (26.7) | 0.102a |
| Neurologic disease | 20 (29.0) | 7 (46.7) | 0.226a |
| Cause of mechanical ventilation | |||
| Pneumonia | 36 (52.2) | 9 (60.0) | 0.776 |
| Airway disease | 9 (13.0) | 4 (26.7) | 0.235a |
| Hemoptysis | 3 (4.3) | 0 | 1.000a |
| Drug intoxication | 13 (18.8) | 1 (6.7) | 0.447a |
| Post operation | 2 (2.9) | 0 | 1.000a |
| Heat failure | 3 (4.3) | 0 | 1.000a |
| Others | 3 (4.3) | 1 (6.7) | 0.552a |
| Type of respiratory failure at intubationb | |||
| Tachypneic respiratory failure | 5 (7.2) | 1 (6.7) | 1.000a |
| Hypercapnic respiratory failure | 31 (44.9) | 7 (46.7) | 0.902 |
| Hypoxic respiratory failure | 22 (31.9) | 1 (6.7) | 0.058a |
| Othersc | 15 (21.7) | 7 (46.7) | 0.058a |
| Severity index | |||
| APACHE II score at ICU admission | 22.0 (17.50–25.00) | 22.00 (19.00–25.00) | 0.516 |
| APACHE II score at extubation | 17.0 (15.0–19.0) | 16.00 (13.00–22.00) | 0.541 |
| Vital sign and arterial blood gas before intubation | |||
| Heart rate | 100.00 (84.00–124.50) | 101.00 (88.00–118.00) | 0.820 |
| Respiratory rate | 22.00 (18.00–26.00) | 22.00 (18.00–28.00) | 0.977 |
| PaCO2 (mmHg) | 44.90 (30.50–60.95) | 38.60 (32.00–86.00) | 0.356 |
| PaO2/FiO2 (mmHg) | 131.97 (86.90–256.19) | 120.86 (80.12–291.90) | 0.907 |
| (A–a) DO2 | 186.97 (48.71–411.96) | 179.53 (50.43- 465.58) | 0.532 |
| Vital sign and arterial blood gas before extubation | |||
| Heart rate on ventilation | 82.00 (73.50–101.00) | 87.00 (76.00–109.00) | 0.272 |
| Respiratory rate on ventilation | 18.00 (16.00–21.00) | 18.00 (15.00–20.00) | 0.366 |
| PaCO2 on ventilation (mmHg) | 34.60 (30.50–40.30) | 35.40 (27.70–46.80) | 0.717 |
| PaO2/FiO2 on ventilation (mmHg) | 286.75 (215.62–361.00) | 321.00 (165.75–400.00) | 0.939 |
| (A–a) DO2 at spontaneous breathing trial | 143.62 (110.02–166.10) | 123.42 (92.25–178.92) | 0.640 |
| High risk patient | |||
| Age older than 65 years | 53 (76.8) | 12 (80.0) | 1.000a |
| Body mass index higher than 30 kg/m2 | 6 (8.7) | 1 (6.7) | 1.000a |
| Ventilator duration more than 7 days | 22 (31.9) | 8 (53.3) | 0.116 |
| Charlson comorbidity index of 2 or more | 26 (37.7) | 7 (46.7) | 0.518a |
| APACHE II score of more than 12 | 66 (95.7) | 14 (93.3) | 0.552a |
| Heart failure as a cause of intubation | 3 (4.3) | 0 | 1.000a |
| Moderate to severe COPD | 15 (21.7) | 1 (6.7) | 0.282a |
| Failure with first SBT trial | 40 (58.0) | 12 (80.0) | 0.111 |
| Duration of mechanical ventilation before extubation (hr) | 117.15 (70.71–210.29) | 181.46 (82.83–273.66) | 0.197 |
| Hospital day before extubation trial (day) | 6.00 (4.00–10.00) | 9.00 (8.00–14.00) | 0.036 |
Values are presented as number (%) or median (interquartile range).
HFNC: high-flow nasal cannula; APACHE: Acute Physiologic and Chronic Health Evaluation; ICU: intensive care unit; PaCO2: arterial partial pressure of carbon dioxide; PaO2/FiO2: ratio of arterial oxygen partial pressure to fractional inspired oxygen; (A–a) DO2: alveolar–arterial oxygen difference; COPD: chronic obstructive pulmonary disease; SBT: spontaneous breathing trial.
DISCUSSION
Table 4.
| Study | Study’s characteristics | Patient’s characteristics | Control | Reintubation | PERF | Physiologic aspect |
|---|---|---|---|---|---|---|
| Futier et al. [13] | Prospective RCT | Surgical patient after major abdominal surgery | LFOS | No difference | No difference | - |
| Dhillon et al. [16] | Retrospective | Critically ill surgical patient | LFOS | No differencea | - | - |
| Yu et al. [14] | Prospective RCT | Surgical patient after thoracoscopic lobectomy | LFOS | Less reintubation in HFNC | Less hypoxemic respiratory failure in HFNC | Better oxygenation, reduction of respiratory rate in HFNC |
| Hernández et al. [6] | Prospective RCT | High risk | NIV | Not inferior in HFNC | Not inferior in HFNC | No difference |
| Yoo et al. [5] | Retrospective | Mixed risk | NIV | No difference | - | - |
| Maggiore et al. [3] | Prospective RCT | Mixed risk | LFOSb | Less reintubation in HFNC | Less PERF in HFNC | Better oxygenation, reduction of respiratory rate in HFNC |
| Hernández et al. [4] | Prospective RCT | Low risk | LFOS | Less reintubation in HFNC | Less PERF in HFNC | No difference |
| Fernandez et al. [12] | Prospective RCT | Mixed risk, but include only hypercapnic patient | LFOS | No difference | No difference | - |
| Song et al. [15] | Prospective RCT | Mixed risk | LFOS | No difference | - | Better oxygenation, reduction of respiratory rate in HFNC |
| This study | Retrospective | High risk | LFOS | No difference | No difference | No differencec |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download