Abstract
Background
This study assessed the association between the initial Acute Physiology and Chronic Health Evaluation (APACHE) II score and good neurological outcome in comatose survivors of out-of-hospital cardiac arrest who received targeted temperature management (TTM).
Methods
Data from survivors of cardiac arrest who received TTM between January 2011 and June 2016 were retrospectively analyzed. The initial APACHE II score was determined using the data immediately collected after return of spontaneous circulation rather than within 24 hours after being admitted to the intensive care unit. Good neurological outcome, defined as Cerebral Performance Category 1 or 2 on day 28, was the primary outcome of this study.
Results
Among 143 survivors of cardiac arrest who received TTM, 62 (43.4%) survived, and 34 (23.8%) exhibited good neurological outcome on day 28. The initial APACHE II score was significantly lower in the patients with good neurological outcome than in those with poor neurological outcome (23.71 ± 4.39 vs. 27.62 ± 6.16, P = 0.001). The predictive ability of the initial APACHE II score for good neurological outcome, assessed using the area under the receiver operating characteristic curve, was 0.697 (95% confidence interval [CI], 0.599 to 0.795; P = 0.001). The initial APACHE II score was associated with good neurological outcome after adjusting for confounders (odds ratio, 0.878; 95% CI, 0.792 to 0.974; P = 0.014).
After resuscitation from out-of-hospital cardiac arrest (OHCA), patients can suffer from systemic inflammatory reactions such as sepsis due to global ischemic injury followed by cardiac arrest and reperfusion injury after return of spontaneous circulation (ROSC) [1,2]. Ischemia/reperfusion injury to the whole body induces generalized activation of coagulation and immunological pathways. These processes increase the risk of multiple organ failure [3]. Among postcardiac arrest patients, 66% had multiple organ failure based on the 3–4 score determined using Sequential Organ Failure Assessment (SOFA) of two or more extracerebral organ systems, and 44% showed worsening organ dysfunction over the first 72 hours after ROSC [1]. A few recent studies have found that the severity of organ dysfunction determined by SOFA score and the Acute Physiology and Chronic Health Evaluation (APACHE) II score were associated with in-hospital mortality in patients after cardiac arrest [4-8]. However, in patients who receive targeted temperature management (TTM), data are lacking on whether these disease severity scores are associated with good neurological outcome, which is the ultimate treatment goal for patients resuscitated after OHCA. Moreover, early identification of good neurological outcome is helpful to physicians so that valuable medical resources and management such as extracorporeal membrane oxygenation (ECMO) or coronary artery bypass graft can be appropriately allocated.
Therefore, in the present study, the initial APACHE II score was calculated based on the data collected immediately after ROSC rather than within 24 hours after being admitted to the intensive care unit (ICU). We investigated the association between initial APACHE II score and good neurological outcome in comatose survivors of OHCA who were treated with TTM.
Data from cardiac arrest survivors treated with TTM at Asan Medical Center between January 2011 and June 2016 were retrospectively analyzed. The Institutional Review Board of the hospital (IRB No. 2016-0476) approved the study. Due to the retrospective nature of this study, prior consent was not required. The study cohort included nontraumatic OHCA survivors 18 years of age or older who had neurological impairment immediately after ROSC and who were treated with TTM.
The collected data included patient demographic information (including age and sex), comorbid conditions, all variables necessary for computing APACHE II scores, and Cerebral Performance Category (CPC) score on day 28. Initial APACHE II score was calculated based on the data collected immediately after ROSC rather than within 24 hours of admission to the ICU. The primary outcome was good neurological one, defined as CPC 1 or 2 on day 28. CPC 1 indicates good cerebral performance (able to work and normal life); CPC 2, moderate cerebral disability (disabled but independent); CPC 3, severe cerebral disability (conscious but disabled and dependent); CPC 4, comatose or in a persistent vegetative state (unconsciousness); CPC 5, brain death/dead [9]. CPC scores were divided into good (CPC 1 or 2) neurological outcome and poor (CPC 3, 4, or 5) neurological outcome, which are accepted categorizations for evaluating post-cardiac arrest patients [10].
Data for the continuous variables are expressed as mean ± standard deviation when following the normal distribution and as intermediate value by quadrant when following the non-normal distribution. Categorical data are expressed in absolute number and percentage. The differences between the continuous variables were analyzed using the t-test, and the differences between the categorical variables were analyzed using the chi-square test. The predictability of the model based on the initial APACHE II score was assessed using the area under the receiver operating characteristic curve (AUROC). Independent contributions to good neurological outcome by variables useful at the time of admission were analyzed with multivariate analysis using a logistic regression model. A P-value less than 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS version 21.0 (IBM Corp., Armonk, NY, USA).
During the study period, 143 patients received TTM after cardiac arrest; 62 patients (43.4%) survived, and 34 (23.8%) had good neurological outcomes on day 28. Table 1 shows the characteristics and differences of the patient groups based on good or poor neurological outcome classified by the CPC score on day 28. Significant differences were observed in age, initial rhythm, arrest cause, and part of the comorbidity between the cardiac arrest survivors receiving TTM who experienced good and poor neurological outcomes. The initial APACHE II score was significantly lower in those with good neurological outcome than in those with poor neurological outcome (23.71 ± 4.39 vs. 27.62 ± 6.16, P = 0.001). Serum lactic acid, C-reactive protein, and creatinine levels showed significant differences between these two groups (Table 2).
The predictive ability of the initial APACHE II score for good neurological outcome assessed using the AUROC was 0.697 (95% confidence interval [CI], 0.599 to 0.795; P = 0.001) (Figure 1). Initial APACHE II score, age, nonshockable rhythm, initial lactate, and comorbid diseases including hypertension, chronic kidney disease, and chronic lung disease were statistically significant in the univariate analysis. In the multivariate analysis, the initial APACHE II score (odds ratio [OR], 0.878; 95% CI, 0.792 to 0.974; P = 0.014), initial lactate (OR, 0.839; 95% CI, 0.731 to 0.963; P = 0.012), and nonshockable rhythm (OR, 0.289; 95% CI, 0.113 to 0.740; P = 0.010) were significantly associated with good neurological outcome (Table 3).
This study evaluated the initial APACHE II scores of OHCA survivors who received TTM and the predictive power of the APACHE II scores for poor neurological outcome evaluated on day 28. The APACHE II score was significantly lower in patients with good neurological outcome than in those with poor neurological outcome. The predictive ability of the initial APACHE II score for good neurological outcome assessed using the AUROC was 0.697. Furthermore, after adjustment for confounders, the initial APACHE II score was associated with good neurological outcome (OR, 0.878; 95% CI, 0.792 to 0.974; P = 0.014). These results indicate that the APACHE II score measured early after ROSC may be useful for predicting neurological outcomes.
The APACHE II score was first introduced in 1985 by Knaus et al. [11] as a method for assessing disease severity. Currently, the APACHE II score is the most widely used method for predicting mortality in ICU patients. Although the APACHE II score is known to be useful for predicting morbidity and mortality in patients after cardiac arrest [8,12], to the best of our knowledge, only a few previous studies specifically validated its use in OHCA survivors who received TTM. Donnino et al. [8] evaluated the APACHE II score in a population of post-cardiac arrest patients, including cases of both in-hospital cardiac arrest and OHCA, and found that the initial APACHE II score in OHCA patients was a modest predictor of poor neurological outcome (AUC = 0.58). However, in the present study, an AUROC of 0.697 was found, indicating better predictive power. This result may be due to differences in the study population because 46% of patients were not treated with TTM. Moreover, our results may be a better reflection of real-world outcomes in patients who receive TTM [13,14]. In North America and Europe, patients expected to have bad neurological outcome are allowed to have life-supporting treatment withdrawn; thus, the longterm neurological outcome of these patients is unknown. In contrast, in South Korea, a legal prohibition exists against the withdrawal of life-supporting treatment from resuscitated patients. As such, all patients who are resuscitated, even those with poor neurological outcome, receive conservative care until death in the hospital, which allowed us to determine the patients’ neurological outcomes.
The majority of resuscitated cardiac arrest patients have impaired consciousness, and the need for prolonged highly intensive care of survivors who are unlikely to recover from neurological conditions presents an immense burden to healthcare systems, families, and society [15]. Many researchers have attempted to develop effective methods to predict survival and neurological prognosis in patients with cardiac arrest. Several biomarkers, brain imaging methods, and neurophysiologic tests have been studied as prognostic tools [16-19]. However, none of these factors alone are identified as a reliable predictor. In addition, most studies have suggested prognosis based on follow-up rather than predicting prognosis early after ROSC. The data collected at the initial stage immediately after ROSC are limited, and the examination may be insufficient given the initial unstable condition of the patient. However, early prediction of prognosis is more valuable because it can influence the appropriate distribution of medical resources and caregiver decisions.
In the present study, we found that the APACHE II score calculated immediately after cardiac arrest can be used to potentially identify good neurological outcome earlier. Although we do not know the optimal value for predicting good outcome, the value appears to be much higher than what is generally used (good outcome of 23.71 vs. poor outcome of 27.62). Therefore, further research with a larger study sample is needed to identify the optimal APACHE II score.
This study had several limitations. First, this was a retrospective study based on a preliminary cardiac arrest registry, making it impossible to avoid limitations related to data collection and analysis. Second, this was a single-center study, and the results may not be generalizable. Finally, we did not compare the prognostic value of APACHE II score with other well-known severity scoring systems such as SOFA or simplified acute physiology score, which should be the focus of future research.
In the present study, the APACHE II score calculated immediately after cardiac arrest was associated with good neurological outcome. Initial APACHE II score might be useful for early identification of good neurological outcome. It will be necessary to develop and verify a better prognostic method by adding parameters and experimenting with more patients.
REFERENCES
1. Adrie C, Adib-Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002; 106:562–8.

2. Mongardon N, Dumas F, Ricome S, Grimaldi D, Hissem T, Pène F, et al. Postcardiac arrest syndrome: from immediate resuscitation to long-term outcome. Ann Intensive Care. 2011; 1:45.

3. Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008; 118:2452–83.
4. Cour M, Bresson D, Hernu R, Argaud L. SOFA score to assess the severity of the post-cardiac arrest syndrome. Resuscitation. 2016; 102:110–5.

5. Nobile L, Taccone FS, Szakmany T, Sakr Y, Jakob SM, Pellis T, et al. The impact of extracerebral organ failure on outcome of patients after cardiac arrest: an observational study from the ICON database. Crit Care. 2016; 20:368.

6. Rittenberger JC, Tisherman SA, Holm MB, Guyette FX, Callaway CW. An early, novel illness severity score to predict outcome after cardiac arrest. Resuscitation. 2011; 82:1399–404.

7. Andrew E, Nehme Z, Bernard S, Smith K. The influence of comorbidity on survival and long-term outcomes after out-of-hospital cardiac arrest. Resuscitation. 2017; 110:42–7.

8. Donnino MW, Salciccioli JD, Dejam A, Giberson T, Giberson B, Cristia C, et al. APACHE II scoring to predict outcome in post-cardiac arrest. Resuscitation. 2013; 84:651–6.

9. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975; 1:480–4.
10. Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa). Circulation. 2004; 110:3385–97.
11. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985; 13:818–29.
12. Niskanen M, Kari A, Nikki P, Iisalo E, Kaukinen L, Rauhala V, et al. Acute physiology and chronic health evaluation (APACHE II) and Glasgow coma scores as predictors of outcome from intensive care after cardiac arrest. Crit Care Med. 1991; 19:1465–73.

13. Kim YJ, Ahn S, Sohn CH, Seo DW, Lee YS, Lee JH, et al. Long-term neurological outcomes in patients after out-of-hospital cardiac arrest. Resuscitation. 2016; 101:1–5.

14. Kern KB. Usefulness of cardiac arrest centers: extending lifesaving post-resuscitation therapies. The Arizona experience. Circ J. 2015; 79:1156–63.
15. Gray WA, Capone RJ, Most AS. Unsuccessful emergency medical resuscitation: are continued efforts in the emergency department justified? N Engl J Med. 1991; 325:1393–8.
16. Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol. 2010; 67:301–7.

17. Martens P. Serum neuron-specific enolase as a prognostic marker for irreversible brain damage in comatose cardiac arrest survivors. Acad Emerg Med. 1996; 3:126–31.

Figure 1.
The area under the receiver operating characteristic curve (AUROC) for initial Acute Physiology and Chronic Health Evaluation (APACHE) II score to predict good neurologic outcome. AUROC was 0.697 (95% confidence interval, 0.599 to 0.795; P = 0.001).
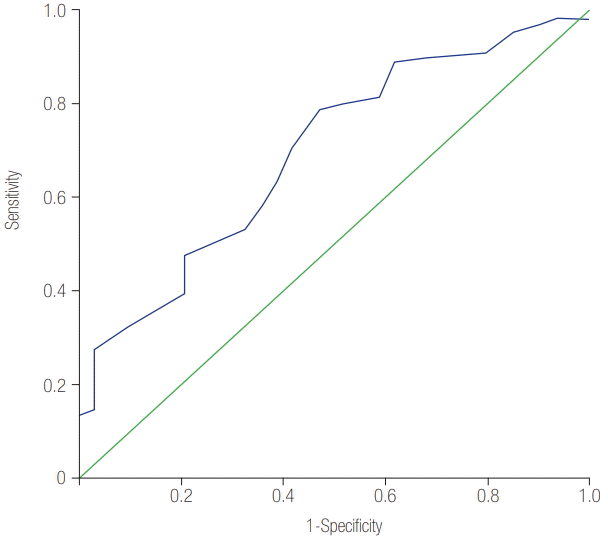
Table 1.
Comparisons of baseline and clinical characteristics
| Variable | All patients (n = 143) | Good neurologic outcome (n = 34) | Poor neurologic outcome (n = 109) | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age (yr) | 59.50 ± 16.4 | 51.88 ± 18.09 | 61.88 ± 15.16 | 0.002 |
| Male sex | 95 (66.4) | 24 (70.6) | 71 (65.1) | 0.788 |
| Initial rhythm | <0.001 | |||
| Shockable rhythm | 36 (25.2) | 17 (47.2) | 19 (52.8) | |
| Nonshockable rhythm | 107 (74.8) | 17 (15.9) | 90 (84.1) | |
| Comorbid condition | ||||
| Coronary artery disease | 20 (14.0) | 2 (5.9) | 18 (16.5) | 0.160 |
| Hypertension | 48 (33.6) | 6 (17.6) | 42 (38.5) | 0.024 |
| Diabetes mellitus | 36 (25.2) | 6 (17.6) | 30 (27.5) | 0.247 |
| Liver cirrhosis | 5 (3.5) | 0 | 5 (4.6) | 0.339 |
| Chronic kidney disease | 15 (10.5) | 0 | 15 (13.8) | 0.022 |
| Chronic lung disease | 18 (12.6) | 0 | 18 (16.5) | 0.007 |
| Stroke | 7 (6.4) | 0 | 7 (6.4) | 0.198 |
| Cardiac arrest cause | <0.001 | |||
| Cardiac | 64 (44.8) | 26 (76.5) | 38 (34.9) | |
| Respiratory | 31 (21.7) | 3 (8.8) | 28 (25.7) | |
| Othersa | 48 (33.6) | 5 (14.7) | 43 (39.4) | |
| Downtime | 28.0 ± 17.0 | 24.7 ± 20.1 | 29.0 ± 15.9 | 0.206 |
| GCS | 3.08 ± 0.49 | 3.29 ± 0.97 | 3.01 ± 0.96 | 0.097 |
| APACHE II score | 26.69 ± 6.01 | 23.71 ± 4.39 | 27.62 ± 6.16 | 0.001 |
Table 2.
Laboratory results of two groups of patients with good or poor neurologic outcome
Table 3.
Multivariate logistic regression analysis demonstrating independent factors associated with the good neurologic outcome in out-of-hospital cardiac arrest patients with TTM
| Variable | OR (95% CI) | P-value |
|---|---|---|
| APACHE II score | 0.878 (0.792–0.974) | 0.014 |
| Nonshockable rhythm | 0.289 (0.113–0.740) | 0.010 |
| Initial lactic acid | 0.839 (0.731–0.963) | 0.012 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download