Abstract
The Jackson table has minimal effects on cardiac function because it does not elevate abdominal and thoracic pressures. In addition, it decreases venous congestion and increases exposure of the surgical field. However, the hips and knees are flexed with inappropriate padding, and venostasis is promoted and increased. Pulmonary thromboembolism (PTE) is fatal; thus immediate diagnosis and treatment are essential. However, clinical signs of intraoperative PTE are difficult to discern. Thrombolytic therapy can be considered as first-line therapy, but bleeding limits its use. The authors report a case of PTE resulting from patient positional change after spine surgery, and the use of immediate postoperative recombinant tissue-type plasminogen activator.
Go to : 
Pulmonary thromboembolism (PTE) presents with various clinical symptoms such as dyspnea, hypoventilation, tachycardia, and hypotension. Morbidity is very high, and immediate diagnosis and treatment are needed, but clinical signs of intraoperative PTE are difficult to discern [1]. Deep vein thrombosis (DVT) is the most common cause of PTE [2], especially in patients in the prone position during spine surgery, a condition during which venous return is decreased and venous flow from the lower extremities is slow. With the use of the Jackson table, inappropriate positioning exacerbates this effect. Thrombolytic therapy can be considered as a first-line therapy for diagnosis of massive PTE during surgery or postoperatively, but complications such as intracranial bleeding limit its use [3]. The authors report a case of life-threatening PTE occurring during position change after spine surgery that was successfully treated with thrombolytic agents.
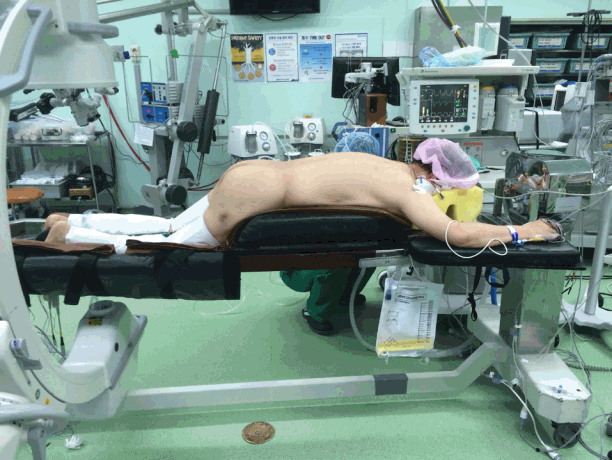
A 62-year-old woman (height, 150.4 cm; weight, 71.1 kg; body mass index [BMI], 31.4 kg/m2) was scheduled to undergo L4–L5–S1 posterior fusion for L5–S1 spondylolisthesis. Past medical history and preoperative evaluations (electrocardiography [ECG], chest radiography, conventional laboratory exams) were nonspecific. Preoperational echocardiography was not performed. The patient’s pain was limited to her back and left leg without restrictions in normal daily activities. Vital signs upon entering the operating room (ECG, pulse, oxygen saturation, bispectral index [BIS] monitoring, and noninvasive blood pressure) were within normal limits. Anesthesia was maintained with desflurane and remifentanil while BIS scores were maintained between 40 and 60. The right radial artery and right internal jugular vein were accessed for invasive blood monitoring and central venous pressure monitoring, respectively. The patient was placed in the prone position on the Jackson table. With this positioning, to maintain the spine in a kyphotic position, the hip and knee joints were slightly flexed (Figure 1). The duration of the operation was 6 hours, without any specific events. The total amount of crystalloid infused was 3,800 ml, and urine output was 300 ml. Blood loss was limited.
After surgery, the patient was rotated to the supine position on a gurney, at which point heart rate (HR) dropped to 43 beats/min, and systolic blood pressure (SBP) was measured at 50 mmHg. Oxygen saturation remained 100%. Considering this decreases transitory decreases resulting from position change, ephedrine 10 mg and atropine 0.5 mg were sequentially administered, but these were ineffective. Immediate blood gas analysis revealed pH 7.21, PaCO2 39 mmHg, PaO2 253 mmHg, bicarbonate 15.6 mmol/L, and SaO2 100%. SBP decreased to 33 mmHg, at which time ephedrine 1 mg was injected following chest compressions. Vital signs temporarily rose to HR 130 beats/min and SBP 110 mmHg, and the patient appeared to respond to verbal commands. However, SBP decreased again, along with a decrease in end tidal CO2 (EtCO2) to 20 mmHg. Oxygen saturation also decreased. Follow-up blood gas analysis revealed pH 6.88, PaCO2 85 mmHg, PaO2 53 mmHg, bicarbonate 15.9 mmol/L, and SaO2 57%. Norepinephrine and dobutamine were continuously infused to increase blood pressure.
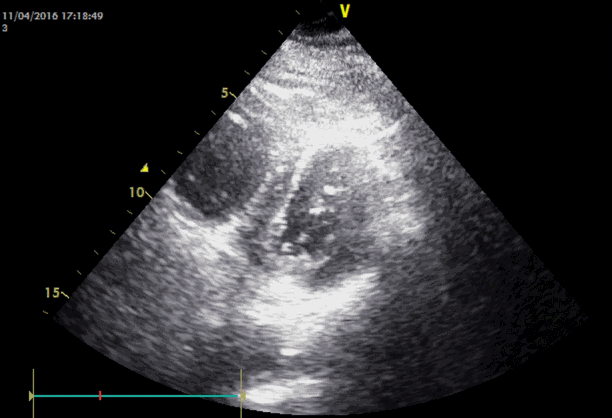
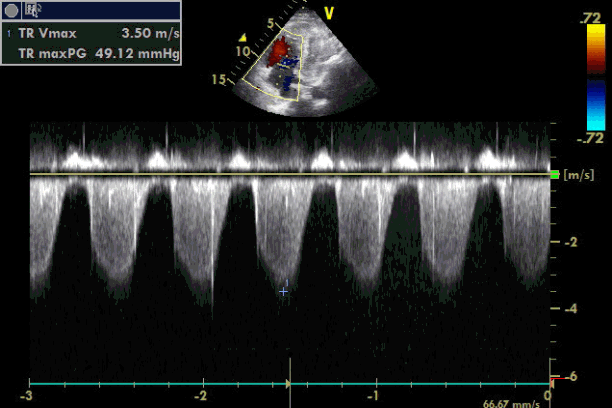
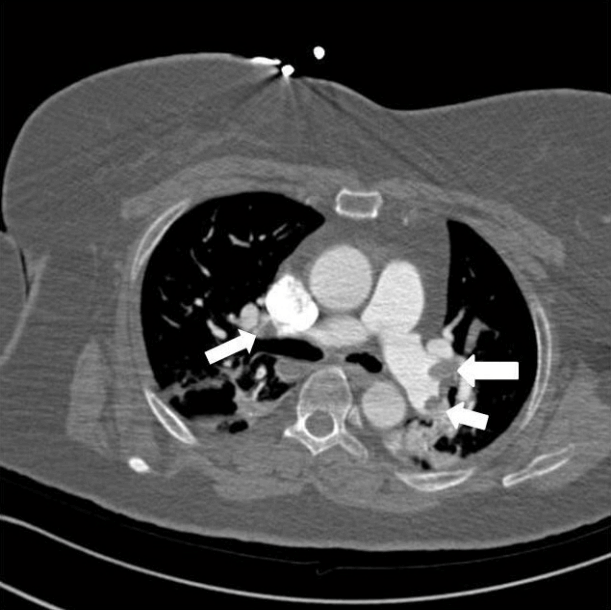
Emergency transesophageal echocardiography (TEE) was performed. The test revealed a hypokinetic dilated right ventricle (RV) and a D-shaped left ventricle (LV) (Figure 2). LV systolic dysfunction was noted without inferior vena cava (IVC) collapsibility. Tricuspid regurgitation was found with 49 mmHg pressure gradient (Figure 3). Considering the decreased EtCO2, elevated PaCO2, and TEE findings, PTE was suspected. Cardiopulmonary resuscitation until return of spontaneous circulation was repeated 3 times. HR was maintained at 120 beats/min, SBP at 150 mmHg, and SpO2 at 70%. Following consultation with cardiology and neurosurgery, we decided to use thrombolytics after diagnosis by multidetector computed tomography (MDCT). MDCT revealed occlusion of both pulmonary arteries with multiple pulmonary thromboembolisms and atelectasis in both lungs, consistent with a diagnosis of acute PTE (Figure 4). DVT was not observed elsewhere. The D-dimer level from a sample obtained in the operating room was above 3,200 ng/ml and troponin I was 0.02 ng/ml.
The patient was transferred to the intensive care unit where treatment with recombinant tissue-type plasminogen activator (tPA) 100 mg (Actilyse, Boehringer Ingelheim, Ingelheim, Germany) was initiated. Weighing the risk and benefits of massive bleeding and the treatment of life-threatening PTE after administration of tPA, massive bleeding can be controlled with various drugs in addition to transfusion. It would be possible to approximate the amount of bleeding and remove any hematomas using surgical drains. In this case, acute administration of thrombolytics was the only option to improve the patient’s hemodynamics. Upon tPA injection, SpO2 dramatically increased to 100%. The patient was placed under ventilator care, in pressure-controlled ventilation mode with peak inspiratory pressure 10 cmH2O, respiration rate 16/min, FiO2 60%, and positive end expiratory pressure 12 cmH2O. The patient’s mental state was drowsy, and norepinephrine 0.1 μg/kg/min and epinephrine 0.05 μg/kg/min were continuously infused to maintain SBP. Within 1 hour of tPA injection, 1,870 ml and 270 ml of blood (total 2,140 ml) were drained from the operation site through two separate drains, and tPA was discontinued. A total of 50 mg of tPA was injected. Over the next 6 hours, 2,000 ml of bleeding was observed, and a total of seven packs of packed red cells and nine packs of fresh frozen plasma were transfused. Bleeding had drastically decreased 6 hours after tPA termination, at which time lab results showed hemoglobin 9.6 g/dl and hematocrit 29.8%.
On postoperative day (POD) 1, epinephrine was discontinued and only norepinephrine was maintained. Lab results showed continued elevation of D-dimer >3,200 mmol/L. Bleeding through the drains had decreased drastically to 130 ml. Cardiac echocardiography continued to show right heart dysfunction, but the size of the LV was clearly reduced compared to the day before. Norepinephrine was discontinued on POD 2, and SBP and HR were stabilized. Chest radiography showed pulmonary edema, which was treated with furosemide. For PTE prevention, enoxaparin 70 mg every 12 hours was administered subcutaneously. Pro-brain natriuretic peptide (pro-BNP) was very high on POD 3 (3,714 pg/ml), and extubation was performed on POD 4 and oxygenation was performed with a high-flow nasal cannula 50 L/min, 60% O2. CT findings on POD 6 revealed improvement of the PTE but not complete resolution. Enoxaparin was discontinued on POD 9 and changed to rivaroxaban. As no further bleeding was observed, the patient was then transferred to the ward.
Go to : 
Placing the patient in the prone position during a posterior approach to the spine allows a better surgical view, but this position is associated with numerous complications. The position itself is difficult to attain, and there are risks of misplacement or malpositioning of anesthetic monitoring lines, intravenous lines, and endotracheal tubes from position change after anesthetic induction. The risks of ulcers, blindness, or brachial plexus injury increase. Tidal volume also decreases from increased inspiratory pressure resulting from compression of the chest. Compression of the abdomen also elevates abdominal pressure, decreasing venous return to the heart and increasing systemic and pulmonary vascular resistance. Furthermore, if the hips and knees are flexed in a knee-chest position, decreased venous return from the lower extremities may lead to the formation of thrombi and related complications. Thus, positioning during lumbar spinal surgery depends on appropriate weight distribution and avoiding extreme joint flexion to prevent DVT and PTE [4].
Tominaga et al. [5] reported a 25% incidence of DVT after spine surgery based on cardiac echocardiography, while Takahashi et al. [6] found a 19% incidence of DVT based on CT. Hohl et al. [7] reported an incidence of 0.88% (51/5,766) of symptomatic PTE after thoracolumbar spine surgery. Lower extremity DVT is the source of 80%-90% of postoperative PTE [2]. Pulmonary embolism (PE) usually forms around 5 days postoperatively rather than immediately after surgery [8], but length of immobility or mechanical stress such as leg elevation and tourniquet inflation also play a role in PE formation [9,10].
There are many predisposing factors for PTE including cancer, pregnancy, hormone replacement therapy, infection, major trauma, limb fracture, surgery, etc [11]. However, in this particular case, the patient was fairly healthy. She was slightly obese, but with BMI <40 kg/m2, the risk of DVT was low. For these reasons, preoperative evaluations specific for PTE (echocardiography, MDCT, and laboratory tests of D-dimer, troponin, BNP) were not performed.
PE was found on postoperative CT, but without a core thrombus of origin, we believe that a thrombus was formed during position change. The use of the Jackson table is preferred because there is no abdominal compression, thus decreasing venous congestion and increasing exposure of the surgical field. In addition, because it does not elevate thoracic pressure and supports the chest and pelvis, cardiac effects are minimized [12]. However, because the hips and knees are flexed and positioned below the heart to promote kyphosis of the surgical area, venostasis is promoted and increased with the inguinal area compressed. Furthermore, long durations of surgery over 6 hours also contribute to risk factors for DVT development.
Other possible causes are pulmonary fat or bone-marrow embolism and intraoperative use of hemostatic agents. Sudden perioperative cardiopulmonary dysfunction as a result of fat embolism is a well-known complication of bone and joint surgery. Takahashi et al. [13] reported that the incidence of moderate to severe embolic events is 80% in spinal instrumental surgery, especially surgery associated with insertion of pedicle screws. There are several cases of PTE associated with application of a hemostatic matrix on the operative field [14]. In this case, a hemostatic gelatin sponge (Cutanplast; Mascia Brunelli, Milan, Italy) was used for hemostasis; no cases of PTE have yet been reported with the use of this device. Nevertheless, DVT can be suspected as a cause of hemodynamic collapse in this case considering the prompt response to tPA.
PTE can present with various clinical signs and is even more difficult to detect in patients under general anesthesia, but it can be diagnosed through changes in monitored parameters. Such findings would be hypotension, sudden decrease in EtCO2, increase in (Pa-Et)CO2 difference, expansion of the jugular vein, and changes in ECG (arrhythmia, ST depression, or right bundle branch block). Wheezing or decreased lung sounds can be heard in both lungs. Extremely high pulmonary artery pressure and central venous pressure may be observed. However, these signs are not restricted to PTE, and because they are nonspecific, other tests should be conducted to confirm this diagnosis.
First, laboratory tests for D-dimer must be performed, along with tests for troponin, BNP, etc. If the results for D-dimer are negative, PTE can be excluded. In addition, MDCT is a noninvasive, simple, and confirmatory diagnostic test. It can diagnose the functioning of structures other than the pulmonary vessels, such as the heart, and can predict risks [15]. If the image resolution is unsatisfactory, pulmonary artery angiography can be performed. However, because this is an invasive procedure, MDCT is generally performed instead. A perfusion diffusion scan demonstrates perfusion of the lung region where the PTE is located but no diffusion. The use of this modality is decreasing, but it can be used in patients allergic to contrast or those who are pregnant. In addition, lower extremity Doppler ultrasonography can be used to assist in the diagnosis of DVT in diagnosing PTE. However, negative findings do not allow the exclusion of PTE.
Cardiac echocardiography is not routinely performed in low-risk patients who are hemodynamically stable, but it is one of the most useful bedside tests in high risk patients who are hypotensive or have cardiogenic shock [16]. Transthoracic echocardiography (TTE) will show signs of RV dysfunction due to elevated LV pressure from PTE. Because the RV wall is thin and compliant, it cannot manage elevated pulmonary vascular resistance and will deviate the ventricular septum to the left. This is an indication for the use of thrombolytics [17]. However, the fact that PTE cannot be found on TTE does not mean that PTE cannot be excluded; a comprehensive decision must be made based upon hemodynamic and other diagnostic findings.
The treatment of PTE remains controversial, and decisions should be based on the degree of signs and hemodynamic variables upon the diagnosis of PTE. When massive PTE results in hemodynamic instability, thrombolytics, surgical embolectomy, and IVC filters, among other approaches, can be used. In this case, interventional embolectomy could not be immediately performed, and because drains were placed in the surgical site, the overall ratio of risks to benefits to the patient should be considered when deciding whether to use thrombolytics. The best results are obtained if tPA is used within 48 hours of the first signs, and the treatment can be effective up to 2 weeks [18]. It is especially effective in patients who show signs of hemodynamic instability due to RV dysfunction, and can decrease mortality or recurrent PTE compared to heparin administration alone. However, its use is limited because bleeding remains a complication. In a study of the use of thrombolytic therapy in 312 patients, 1.9% developed intracranial hemorrhage [3]. In our case, we decided to use thrombolytics due to hemodynamic instability and minimal postoperative bleeding. However, massive bleeding occurred with the use of tPA, and thus it was discontinued within 1 hour and replaced with heparinization. Postoperative site bleeding was increased due to the use of tPA, but no other bleeding foci were found. Decrease in PTE was also observed, and hemodynamic parameters were stabilized with its use.
Patients taking antithrombotic or antiplatelet agents should discontinue medication in the preoperative period and resume treatment soon after the operation. However, determination of proper resumption presents a dilemma due to bleeding risk. Recent guidelines recommend resuming warfarin approximately 12 to 24 hours after surgery, and the prompt resumption of antiplatelet agents after surgery. For patients receiving bridging anticoagulation with low-molecular-weight heparin (LWMH), the guidelines suggest resuming LWMH 48 to 72 hours after surgery with high bleeding risk and approximately 24 hours after surgery with low bleeding risk [19,20].
Anesthesiologists must keep in mind that any patient undergoing surgery can develop PTE. Placement in a prone position for an extended period of time can be a high risk factor for PTE, and if this is suspected, immediate diagnosis and treatment should be performed. Furthermore, considering the high mortality in patients who develop PTE, preventive measures are necessary in high-risk patients. Prevention with preoperative and postoperative heparin administration, lower extremity compression stockings, and early postoperative ambulation can be considered as well. Furthermore, a variety of treatment approaches should be considered, taking into account individual patients’ hemodynamic parameters and weighing risks and benefits.
Go to : 
REFERENCES
1. Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002; 121:877–905.
2. Schizas C, Neumayer F, Kosmopoulos V. Incidence and management of pulmonary embolism following spinal surgery occurring while under chemical thromboprophylaxis. Eur Spine J. 2008; 17:970–4.

3. Kanter DS, Mikkola KM, Patel SR, Parker JA, Goldhaber SZ. Thrombolytic therapy for pulmonary embolism: frequency of intracranial hemorrhage and associated risk factors. Chest. 1997; 111:1241–5.
4. Nicol M, Sun Y, Craig N, Wardlaw D. Incidence of thromboembolic complications in lumbar spinal surgery in 1,111 patients. Eur Spine J. 2009; 18:1548–52.

5. Tominaga H, Setoguchi T, Tanabe F, Kawamura I, Tsuneyoshi Y, Kawabata N, et al. Risk factors for venous thromboembolism after spine surgery. Medicine (Baltimore). 2015; 94:e466.

6. Takahashi H, Yokoyama Y, Iida Y, Terashima F, Hasegawa K, Saito T, et al. Incidence of venous thromboembolism after spine surgery. J Orthop Sci. 2012; 17:114–7.

7. Hohl JB, Lee JY, Rayappa SP, Nabb CE, Devin CJ, Kang JD, et al. Prevalence of venous thromboembolic events after elective major thoracolumbar degenerative spine surgery. J Spinal Disord Tech. 2015; 28:E310–5.

8. Bohl DD, Webb ML, Lukasiewicz AM, Samuel AM, Basques BA, Ahn J, et al. Timing of complications after spinal fusion surgery. Spine (Phila Pa 1976). 2015; 40:1527–35.

9. Jang IS, Kim HT, An SK, Kwon YE, Lee JH. Pulmonary thromboembolism occurred immediately after leg elevation under induction of general anesthesia in a patient with femur fracture: a case report. Anesth Pain Med. 2009; 4:129–32.
10. Song JE, Chun DH, Shin JH, Park C, Lee JY. Pulmonary thromboembolism after tourniquet inflation under spinal anesthesia: a case report. Korean J Anesthesiol. 2010; 59(Suppl):S82–5.

11. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014; 35:3033–69.
12. Dharmavaram S, Jellish WS, Nockels RP, Shea J, Mehmood R, Ghanayem A, et al. Effect of prone positioning systems on hemodynamic and cardiac function during lumbar spine surgery: an echocardiographic study. Spine (Phila Pa 1976). 2006; 31:1388–93.

13. Takahashi S, Kitagawa H, Ishii T. Intraoperative pulmonary embolism during spinal instrumentation surgery. A prospective study using transoesophageal echocardiography. J Bone Joint Surf Br. 2003; 85:90–4.
14. Steinestel K, Geiger A, Naraghi R, Kunz U, Danz B, Kraft K, et al. Fatal thromboembolism to the left pulmonary artery by locally applied hemostatic matrix after surgical removal of spinal schwannoma: a case report. Hum Pathol. 2013; 44:294–8.

15. Schaefer-Prokop C, Prokop M. MDCT for the diagnosis of acute pulmonary embolism. Eur Radiol. 2005; 15 Suppl 4:D37–41.

16. Vieillard-Baron A, Qanadli SD, Antakly Y, Fourme T, Loubières Y, Jardin F, et al. Transesophageal echocardiography for the diagnosis of pulmonary embolism with acute cor pulmonale: a comparison with radiological procedures. Intensive Care Med. 1998; 24:429–33.

17. Kucher N, Goldhaber SZ. Cardiac biomarkers for risk stratification of patients with acute pulmonary embolism. Circulation. 2003; 108:2191–4.

18. Daniels LB, Parker JA, Patel SR, Grodstein F, Goldhaber SZ. Relation of duration of symptoms with response to thrombolytic therapy in pulmonary embolism. Am J Cardiol. 1997; 80:184–8.

19. Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141(2 Suppl):e326S–e350S.
20. Darvish-Kazem S, Gandhi M, Marcucci M, Douketis JD. Perioperative management of antiplatelet therapy in patients with a coronary stent who need noncardiac surgery: a systematic review of clinical practice guidelines. Chest. 2013; 144:1848–56.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download