Glufosinate ammonium (GA) is a commercial herbicide that causes several neurologic complications such as loss of consciousness, convulsions, and memory impairment [1]. The N-methyl-D-aspartate (NMDA)-type glutamate receptor in the hippocampus is the main target for GA, and its binding to this receptor is implicated in the toxic anterograde amnesia effects of GA [2,3]. We report a rare but typical case of anterograde amnesia and bilateral hippocampal involvement in acute GA intoxication.
CASE REPORT
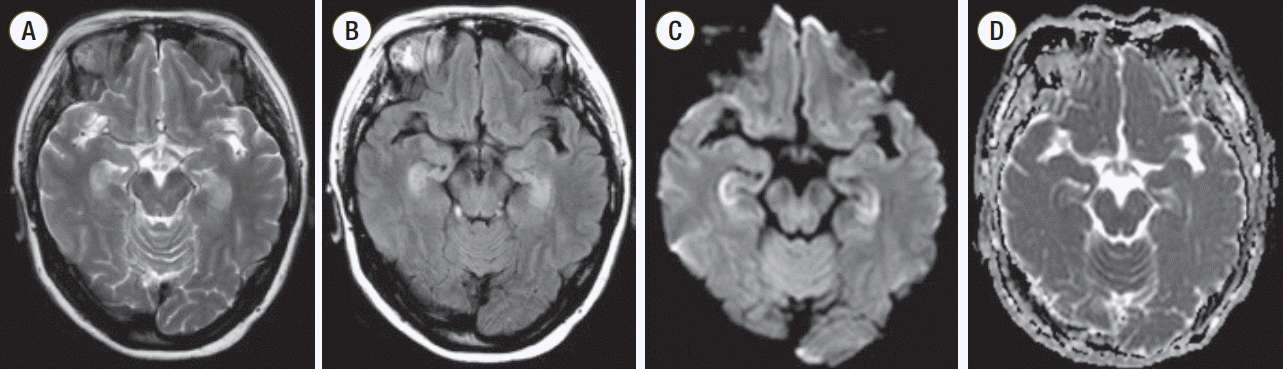
A 53-year-old woman with no previous medical history presented to the emergency department 9 hours after drinking 200 ml of GA and trying to hang herself as a suicide attempt. Formulation of the herbicide was as follows: GA, 18%; alcohol (C12–14), ethoxylated monoether with sulfuric acid, and sodium salts (CAS No. 68909-66-0) as surfactants, 28.5%; antifoaming agent and coloring agent, 0.5%; and water, 53%. When presented to the emergency department, she was slightly drowsy but had no symptoms or signs of gastrointestinal irritation. An external wound-like bruise was not seen on the head or neck. The vital signs were stable. Arterial blood gas determination showed slight metabolic and respiratory acidosis with a pH of 7.31, PO2 of 79.6 mmHg, PCO2 of 46.2 mmHg, and HCO3 of 22.5 mmol/L. The serum ammonium level was 308 μM. The general and neurologic examinations were unremarkable, except for drowsy mentality and increased irritability. She immediately received gastric lavage followed by charcoal and was admitted to the emergency intensive care unit. On hospital day 1, she could not remember that she had been admitted to the hospital. She was unable to remember things she had just been told, but she could recall events of decades ago; she did not understand why she had been admitted to the hospital despite being told repeatedly by her family. She could not recall people who had recently visited her, even the doctors and nurses. On hospital day 4, she became alert and her medical condition was normal except for persistent amnesia. So, she was referred to the Neurology Department for further assessment of her anterograde amnesia. On neurologic examination, motor and sensory function was intact, and extrapyramidal signs and symptoms were evaluated, such as abnormal movement or tonic abnormalities of muscles. On the Mini-Mental State Examination, she scored 24 of 30 points. She lost 2 points in the category of “Orientation to time,” 1 point in the category of “Orientation to place,” and 3 points in the category of “Registration recall.” On hospital day 6, brain magnetic resonance imaging (MRI) was performed and showed symmetric, bilateral increase in signal intensity in the hippocampus on fluid-attenuated inversion recovery and T2-weighted images (Figure 1). On hospital day 8, waking and sleep electroencephalogram was recorded and interpreted as normal for the patient. On hospital day 9, she was discharged with no medical problems except anterograde amnesia. Eleven days after the onset of amnesia, she reported back to the neurology outpatient department, where her anterograde amnesia was evaluated and still present, but was slowly improving.
DISCUSSION
The hippocampus is the main structure in the brain that is responsible for learning and memory. Also, the hippocampus is a plastic and vulnerable structure that can be damaged by a variety of stimuli and is affected by a variety of neurological and psychiatric disorders [4]. In several cases of anterograde amnesia after GA intoxication, brain MRI showed hyper-signal intensity in the hippocampus, and this injury can be explained by overstimulation of the glutamate-NMDA receptor caused by glufosinate, which acts similar to glutamate in the hippocampus [1,5]. This may result in memory loss, especially anterograde amnesia, as in this case. Moreover, the increasing concentration of nitric oxide followed by hyperammonemia causes oxidative stress in the central nervous system. As in our case, hyper-ammonemia in GA intoxication could also lead to injury of the hippocampus and result in amnesia [6-9].
However, cases that show typical anterograde amnesia with a focal insult of the hippocampus on MRI after GA intoxication are very rare. Almost all cases of GA intoxication report general signs and symptoms of generalized tonic-clonic seizure, respiratory hold, severe deterioration in cognitive function, and encephalopathy [10-14]. One reason why this case was different from the other cases in regard to general signs and symptoms is that the GA doses were different. The GA dose in this case study was approximately half those in other case reports. This indicates variability of symptoms according to GA dose, and symptom and toxicity predictions can be made along the course of GA intoxication in patients depending on the dose ingested.
In addition, the hanging attempt could have also contributed to hippocampus injury and resulted in amnesia. Hanging induces ischemic damage in the brain, and the hippocampus is one of the most vulnerable organs in the brain in ischemic injury. In one experiment, pyramidal cells of a CA1 lesion in the hippocampus decreased after intentional hypoxic hanging injury in rats [15]. This decrease in cell count caused by cell death in the hippocampus may have contributed to amnesia symptoms associated with hanging. Considering that behavior and personal changes are much more common in serious hanging incidents due to injuries of watershed cerebral cortex and basal ganglia and more widespread brain damage, the hanging event was likely mild and brief in this case [16].
To the best of our knowledge, there is not an antidote generally used in the treatment of GA intoxication. The neurologic symptoms of GA are mainly explained by overstimulation of NMDA receptors. So, the modulation of NMDA and glutamate may be explored in the development of an antidote for GA intoxication. Also, considering the neurotoxicity of hyperammonemia, procedures for reducing serum ammonia could be applied to GA intoxication patients, similar to those used to treat hyperammonemia in liver failure patients.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download