Abstract
Background and Purpose
Methods
Results
ACKNOWLEDGMENTS
References
Figure 1.
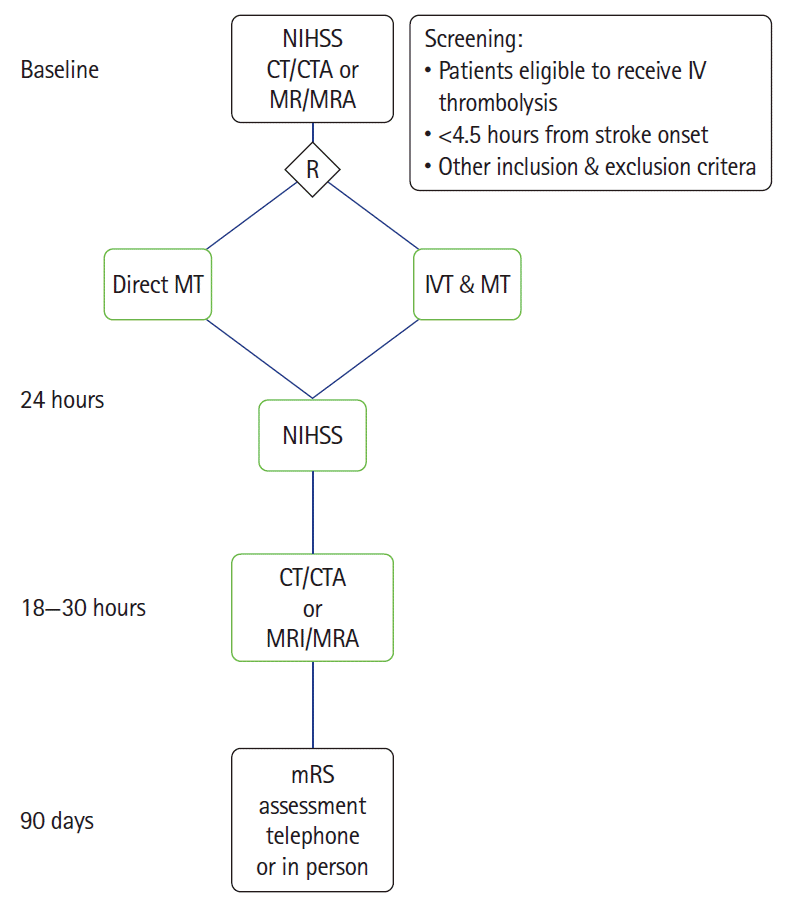
Table 1.
Table 2.
| Characteristic | DIRECT-MT [18] | SKIP [19] | DEVT [20] | MR CLEAN-NO IV [21] | SWIFT-DIRECT [22] | DIRECT-SAFE |
|---|---|---|---|---|---|---|
| Main inclusion criteria | Age ≥18 years | Age ≥18 & <86 years | Age ≥18 years | Age ≥18 years | Age ≥18 years | Age ≥18 years |
| mRS 0 or 1 | mRS 0 or 2 | mRS 0 or 1 | mRS 0 or 1 | mRS 0 or 1 | mRS 0–3 | |
| ICA, MCA-M1 or M2 occlusion on CTA | ICA, or MCA-M1 occlusion on CTA or MRA | ICA, or MCA-M1 occlusion on CTA or MRA | ICA, or MCA-M1 or proximal M2 occlusion on CTA or MRA | ICA, or MCA-M1 occlusion or both on CTA or MRA | ICA, MCA-M1 or M2 or basilar on CTA or MRA | |
| NIHSS: no limit | NIHSS: ≥6 | NIHSS: no limit | NIHSS: ≥2 | NIHSS: ≥5 or <30 | NIHSS: no limit | |
| Onset to IV tPA ≤4.5 hours | Onset to IV tPA ≤4.5 hours | Onset to IV tPA ≤4.5 hours | Onset to randomization ≤4 hours 15 minutes | Onset to IV tPA ≤4.5 hours | ||
| Hospital arrival to puncture ≤90 minutes | ||||||
| Country | China | Japan | China | Europe | North America & Europe | Australia, New Zealand, China, Vietnam |
| Device | Any approved device | Any approved device | Any approved device | Any approved device | Solitaire stent retriever with proximal protection device first | Trevo stent retriever first |
| Thrombolytic | Alteplase, 0.9 mg/kg | Alteplase, 0.6 mg/kg | Alteplase, 0.9 mg/kg | Alteplase, 0.9 mg/kg | Alteplase, 0.9 mg/kg | Alteplase, 0.9 mg/kg OR Tenecteplase 0.25 mg/kg |
| Design | Non-inferiority | Non-inferiority | Non-inferiority | Superiority | Non-inferiority | Non-inferiority |
| Non-inferiority margins | mRS shift with a non-inferiority margin OR 0.80 | mRS 0–2 with a non-inferiority margin OR 0.74 | mRS 0–2 with a risk difference non-inferiority margin 10% | mRS shift with a non-inferiority margin OR 0.80 | mRS 0–2 with a non-inferiority margin 12% | mRS 0–2 or return to baseline with risk difference non-inferiority margin 10% |
| Treatment | MT (n=327) | MT (n=101) | MT (n=116) | MT (n=273) | MT (n=201) | NA |
| IVT before MT (n=329) | IVT before MT (n=103) | IVT before MT (n=118) | IVT before MT (n=266) | IVT before MT (n=207) | ||
| Publication status | 2020 | 2021 | 2021 | Abstract at the International Stroke Congress February 2021 | Abstract at the European Stroke Organization Congress September 2021 | NA |
| Main outcome | Demonstrated non-inferiority | Did not demonstrate non-inferiority | Demonstrated non-inferiority | Did not demonstrate superiority | Did not demonstrate non-inferiority | NA |
DIRECT-MT, Direct Intra-arterial Thrombectomy in Order to Revascularize AIS Patients With Large Vessel Occlusion Efficiently in Chinese Tertiary Hospitals; SKIP, Direct Mechanical Thrombectomy in Acute LVO Stroke; DEVT, Direct Endovascular Thrombectomy vs Combined IVT and Endovascular Thrombectomy for Patients With Acute Large Vessel Occlusion in the Anterior Circulation; MR CLEAN-NO IV, Multicenter Randomized CLinical trial of Endovascular treatment for Acute ischemic stroke in the Netherlands-NO IV; SWIFT-DIRECT, Bridging Thrombolysis Versus Direct Mechanical Thrombectomy in Acute Ischemic Stroke; DIRECT-SAFE, A Randomized Controlled Trial of DIRECT Endovascular Clot Retrieval versus Standard Bridging Therapy; mRS, modified Rankin Scale; ICA, internal carotid artery; MCA, middle cerebral artery; CTA, computed tomography angiography; NIHSS, National Institutes of Health Stroke Scale; IV tPA, intravenous tissue plasminogen activator; MRA, magnetic resonance angiography; MT, mechanical thrombectomy; IVT, intravenous thrombolysis; NA, not applicable.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download