1. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke. A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019; 50:e344–e418.

2. Ko SB, Park HK, Kim BM, Heo JH, Rha JH, Kwon SU, et al. 2019 Update of the Korean clinical practice guidelines of stroke for endovascular recanalization therapy in patients with acute ischemic stroke. J Stroke. 2019; 21:231–240.

3. Baik SK, Oh SJ, Park KP, Lee JH. Intra-arterial tirofiban infusion for partial recanalization with stagnant flow in hyperacute cerebral ischemic stroke. Interv Neuroradiol. 2011; 17:442–451.

4. Kang DH, Yoon W, Kim SK, Baek BH, Lee YY, Kim YW, et al. Endovascular treatment for emergent large vessel occlusion due to severe intracranial atherosclerotic stenosis. J Neurosurg. 2019; 130:1949–1956.

5. Baek JH, Kim BM, Heo JH, Kim DJ, Nam HS, Kim YD. Outcomes of endovascular treatment for acute intracranial atherosclerosis-related large vessel occlusion. Stroke. 2018; 49:2699–2705.

6. Lee JS, Hong JM, Lee KS, Suh HI, Demchuk AM, Hwang YH, et al. Endovascular therapy of cerebral arterial occlusions: intracranial atherosclerosis versus embolism. J Stroke Cerebrovasc Dis. 2015; 24:2074–2080.

7. Jia B, Feng L, Liebeskind DS, Huo X, Gao F, Ma N, et al. Mechanical thrombectomy and rescue therapy for intracranial large artery occlusion with underlying atherosclerosis. J Neurointerv Surg. 2018; 10:746–750.

8. Dobrocky T, Kaesmacher J, Bellwald S, Piechowiak E, Mosimann PJ, Zibold F, et al. Stent-retriever thrombectomy and rescue treatment of M1 occlusions due to underlying intracranial atherosclerotic stenosis: cohort analysis and review of the literature. Cardiovasc Intervent Radiol. 2019; 42:863–872.

9. Kim YW, Hong JM, Park DG, Choi JW, Kang DH, Kim YS, et al. Effect of intracranial atherosclerotic disease on endovascular treatment for patients with acute vertebrobasilar occlusion. AJNR Am J Neuroradiol. 2016; 37:2072–2078.

10. Lee JS, Lee SJ, Yoo JS, Hong JH, Kim CH, Kim YW, et al. Prognosis of acute intracranial atherosclerosis-related occlusion after endovascular treatment. J Stroke. 2018; 20:394–403.

11. Turan TN, Cotsonis G, Lynn MJ, Wooley RH, Swanson S, Williams JE, et al. Intracranial stenosis: impact of randomized trials on treatment preferences of US neurologists and neurointerventionists. Cerebrovasc Dis. 2014; 37:203–211.

12. Zaidat OO, Castonguay AC, Nguyen TN, Becker KJ, Derdeyn CP, Nelson PK, et al. Impact of SAMMPRIS on the future of intracranial atherosclerotic disease management: polling results from the ICAD symposium at the International Stroke Conference. J Neurointerv Surg. 2014; 6:225–230.

13. Kim JG, Suh DC, Song Y, Choi JC, Lee DH. Direct stenting of intracranial atherosclerosis-related acute large vessel occlusion. Clin Neuroradiol. 2021; 31:833–841.

14. Wu C, Chang W, Wu D, Wen C, Zhang J, Xu R, et al. Angioplasty and/or stenting after thrombectomy in patients with underlying intracranial atherosclerotic stenosis. Neuroradiology. 2019; 61:1073–1081.

15. Gross BA, Desai SM, Walker G, Jankowitz BT, Jadhav A, Jovin TG. Balloon-mounted stents for acute intracranial large vessel occlusion secondary to presumed atherosclerotic disease: evolution in an era of supple intermediate catheters. J Neurointerv Surg. 2019; 11:975–978.

16. Feng MT, Zhang HJ, Zhang YX, Xing PF, Zhang L, Zhang YW, et al. Stent angioplasty for acute intracranial atherosclerotic occlusion after failed thrombectomy: a single-institution series of 55 patients. World Neurosurg. 2019; 130:e444–e448.

17. Yang D, Lin M, Wang S, Wang H, Hao Y, Zi W, et al. Primary angioplasty and stenting may be superior to thrombectomy for acute atherosclerotic large-artery occlusion. Interv Neuroradiol. 2018; 24:412–420.

18. Yoon W, Kim SK, Park MS, Kim BC, Kang HK. Endovascular treatment and the outcomes of atherosclerotic intracranial stenosis in patients with hyperacute stroke. Neurosurgery. 2015; 76:680–686.

19. Rai AT, Seldon AE, Boo S, Link PS, Domico JR, Tarabishy AR, et al. A population-based incidence of acute large vessel occlusions and thrombectomy eligible patients indicates significant potential for growth of endovascular stroke therapy in the USA. J Neurointerv Surg. 2017; 9:722–726.

20. Kim JS, Kim YJ, Ahn SH, Kim BJ. Location of cerebral atherosclerosis: why is there a difference between East and West? Int J Stroke. 2018; 13:35–46.

21. Lee D, Lee DH, Suh DC, Kim BJ, Kwon SU, Kwon HS, et al. Endovascular treatment in patients with cerebral artery occlusion of three different etiologies. J Stroke. 2020; 22:234–244.

22. Suh HI, Hong JM, Lee KS, Han M, Choi JW, Kim JS, et al. Imaging predictors for atherosclerosis-related intracranial large artery occlusions in acute anterior circulation stroke. J Stroke. 2016; 18:352–354.

23. Baek JH, Kim BM. Angiographical identification of intracranial, atherosclerosis-related, large vessel occlusion in endovascular treatment. Front Neurol. 2019; 10:298.

24. Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011; 365:993–1003.
25. Zaidat OO, Fitzsimmons BF, Woodward BK, Wang Z, Killer-Oberpfalzer M, Wakhloo A, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA. 2015; 313:1240–1248.
26. Libby P. Inflammation in atherosclerosis. Nature. 2002; 420:868–874.

27. van der Wal AC. Coronary artery pathology. Heart. 2007; 93:1484–1489.

28. Guidelines for the performance of percutaneous transluminal coronary angioplasty. Circulation. 1982; 66:693–694.
29. Iqbal J, Gunn J, Serruys PW. Coronary stents: historical development, current status and future directions. Br Med Bull. 2013; 106:193–211.

30. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011; 124:e574–e651.
31. Lee RM. Morphology of cerebral arteries. Pharmacol Ther. 1995; 66:149–73.

32. Fiorella D, Derdeyn CP, Lynn MJ, Barnwell SL, Hoh BL, Levy EI, et al. Detailed analysis of periprocedural strokes in patients undergoing intracranial stenting in Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS). Stroke. 2012; 43:2682–2688.

33. Khatri R, McKinney AM, Swenson B, Janardhan V. Bloodbrain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology. 2012; 79(13 Suppl 1):S52–S57.

34. Heo JH, Lee KY, Kim SH, Kim DI. Immediate reocclusion following a successful thrombolysis in acute stroke: a pilot study. Neurology. 2003; 60:1684–1687.

35. Zinkstok SM, Beenen LF, Majoie CB, Marquering HA, de Haan RJ, Roos YB. Early deterioration after thrombolysis plus aspirin in acute stroke: a post hoc analysis of the Antiplatelet Therapy in Combination with Recombinant t-PA Thrombolysis in Ischemic Stroke trial. Stroke. 2014; 45:3080–3082.
36. Cappellari M, Turcato G, Forlivesi S, Zivelonghi C, Bovi P, Bonetti B, et al. STARTING-SICH nomogram to predict symptomatic intracerebral hemorrhage after intravenous thrombolysis for stroke. Stroke. 2018; 49:397–404.

37. Tsivgoulis G, Katsanos AH, Mavridis D, Gdovinova Z, Karliński M, Macleod MJ, et al. Intravenous thrombolysis for ischemic stroke patients on dual antiplatelets. Ann Neurol. 2018; 84:89–97.

38. Altersberger VL, Sturzenegger R, Räty S, Hametner C, Scheitz JF, Moulin S, et al. Prior dual antiplatelet therapy and thrombolysis in acute stroke. Ann Neurol. 2020; 88:857–859.

39. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41.

40. Tsang A, Lau KK, Tsang F, Tse M, Lee R, Lui WM. Severity of intracranial carotid artery calcification in intracranial atherosclerosis-related occlusion treated with endovascular thrombectomy. Clin Neurol Neurosurg. 2018; 174:214–216.

41. Baek JH, Kim BM, Kim JW, Kim DJ, Heo JH, Nam HS, et al. Utility of leptomeningeal collaterals in predicting intracranial atherosclerosis-related large vessel occlusion in endovascular treatment. J Clin Med. 2020; 9:2784.

42. Baek JH, Kim BM, Kim DJ, Heo JH, Nam HS, Song D, et al. Importance of truncal-type occlusion in stentriever-based thrombectomy for acute stroke. Neurology. 2016; 87:1542–1550.

43. Baek JH, Kim BM, Yoo J, Nam HS, Kim YD, Kim DJ, et al. Predictive value of computed tomography angiography-determined occlusion type in stent retriever thrombectomy. Stroke. 2017; 48:2746–2752.

44. Lee SJ, Hong JM, Choi JW, Kang DH, Kim YW, Kim YS, et al. CTA-based truncal-type occlusion is best matched with postprocedural fixed focal stenosis in vertebrobasilar occlusions. Front Neurol. 2019; 9:1195.

45. Haussen DC, Bouslama M, Dehkharghani S, Grossberg JA, Bianchi N, Bowen M, et al. Automated CT perfusion prediction of large vessel acute stroke from intracranial atherosclerotic disease. Interv Neurol. 2018; 7:334–340.

46. Garcia-Bermejo P, Patro SN, Ahmed AZ, Al Rumaihi G, Akhtar N, Kamran S, et al. Baseline occlusion angiographic appearance on mechanical thrombectomy suggests underlying etiology and outcome. Front Neurol. 2019; 10:499.

47. Jin X, Shi F, Chen Y, Zheng X, Zhang J. Jet-like appearance in angiography as a predictive image marker for the occlusion of intracranial atherosclerotic stenosis. Front Neurol. 2020; 11:575567.

48. Urban P, Macaya C, Rupprecht HJ, Kiemeneij F, Emanuelsson H, Fontanelli A, et al. Randomized evaluation of anticoagulation versus antiplatelet therapy after coronary stent implantation in high-risk patients: the multicenter aspirin and ticlopidine trial after intracoronary stenting (MATTIS). Circulation. 1998; 98:2126–2132.
49. Patti G, Pasceri V, Colonna G, Miglionico M, Fischetti D, Sardella G, et al. Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol. 2007; 49:1272–1278.
50. Husted S, Emanuelsson H, Heptinstall S, Sandset PM, Wickens M, Peters G. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur Heart J. 2006; 27:1038–1047.

51. Bücke P, Aguilar Pérez M, AlMatter M, Hellstern V, Bäzner H, Henkes H. Functional outcome and safety of intracranial thrombectomy after emergent extracranial stenting in acute ischemic stroke due to tandem occlusions. Front Neurol. 2018; 9:940.

52. Yang P, Zhang Y, Zhang L, Zhang Y, Treurniet KM, Chen W, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020; 382:1981–1993.

53. Suzuki K, Matsumaru Y, Takeuchi M, Morimoto M, Kanazawa R, Takayama Y, et al. Effect of mechanical thrombectomy without vs with intravenous thrombolysis on functional outcome among patients with acute ischemic stroke: the SKIP randomized clinical trial. JAMA. 2021; 325:244–253.
54. Zi W, Qiu Z, Li F, Sang H, Wu D, Luo W, et al. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the DEVT randomized clinical trial. JAMA. 2021; 325:234–243.
55. Nogueira RG, Tsivgoulis G. Large vessel occlusion strokes after the DIRECT-MT and SKIP trials: is the alteplase syringe half empty or half full? Stroke. 2020; 51:3182–3186.
56. Jolly SS, James S, Džavík V, Cairns JA, Mahmoud KD, Zijlstra F, et al. Thrombus aspiration in ST-segment-elevation myocardial infarction: an individual patient meta-analysis. Thrombectomy Trialists Collaboration. Circulation. 2017; 135:143–152.

57. Lee JS, Hong JM, Lee KS, Suh HI, Choi JW, Kim SY. Primary stent retrieval for acute intracranial large artery occlusion due to atherosclerotic disease. J Stroke. 2016; 18:96–101.

58. Lee JS, Lee SJ, Hong JM, Choi JW, Yoo J, Hong JH, et al. Solitaire thrombectomy for acute stroke due to intracranial atherosclerosis-related occlusion: ROSE ASSIST study. Front Neurol. 2018; 9:1064.

59. Yoo J, Lee SJ, Hong JH, Kim YW, Hong JM, Kim CH, et al. Immediate effects of first-line thrombectomy devices for intracranial atherosclerosis-related occlusion: stent retriever versus contact aspiration. BMC Neurol. 2020; 20:283.

60. Kang DH, Yoon W, Baek BH, Kim SK, Lee YY, Kim JT, et al. Front-line thrombectomy for acute large-vessel occlusion with underlying severe intracranial stenosis: stent retriever versus contact aspiration. J Neurosurg. 2019; 132:1202–1208.

61. Kang DH, Hwang YH. Frontline contact aspiration treatment for emergent large vessel occlusion: a review focused on practical techniques. J Stroke. 2019; 21:10–22.

62. Tsang A, Orru E, Klostranec JM, Yang IH, Lau KK, Tsang F, et al. Thrombectomy outcomes of intracranial atherosclerosis-related occlusions. Stroke. 2019; 50:1460–1466.

63. Kang DH, Kim YW, Hwang YH, Park SP, Kim YS, Baik SK. Instant reocclusion following mechanical thrombectomy of in situ thromboocclusion and the role of low-dose intra-arterial tirofiban. Cerebrovasc Dis. 2014; 37:350–5.

64. Zhao W, Che R, Shang S, Wu C, Li C, Wu L, et al. Low-dose tirofiban improves functional outcome in acute ischemic stroke patients treated with endovascular thrombectomy. Stroke. 2017; 48:3289–3294.

65. Kim YW, Sohn SI, Yoo J, Hong JH, Kim CH, Kang DH, et al. Local tirofiban infusion for remnant stenosis in large vessel occlusion: tirofiban ASSIST study. BMC Neurol. 2020; 20:284.

66. Woo HG, Sunwoo L, Jung C, Kim BJ, Han MK, Bae HJ, et al. Feasibility of permanent stenting with solitaire FR as a rescue treatment for the reperfusion of acute intracranial artery occlusion. AJNR Am J Neuroradiol. 2018; 39:331–336.

67. Li W, Lin L, Zhang M, Wu Y, Liu C, Li X, et al. Safety and preliminary efficacy of early tirofiban treatment after alteplase in acute ischemic stroke patients. Stroke. 2016; 47:2649–2651.

68. Coller BS. Platelets and thrombolytic therapy. N Engl J Med. 1990; 322:33–42.

69. Adeoye O, Sucharew H, Khoury J, Vagal A, Schmit PA, Ewing I, et al. Combined approach to lysis utilizing eptifibatide and recombinant tissue-type plasminogen activator in acute ischemic stroke-full dose regimen stroke trial. Stroke. 2015; 46:2529–2533.

70. Cervo A, Ferrari F, Barchetti G, Quilici L, Piano M, Boccardi E, et al. Use of cangrelor in cervical and intracranial stenting for the treatment of acute ischemic stroke: a “real life” single-center experience. AJNR Am J Neuroradiol. 2020; 41:2094–2099.

71. Elhorany M, Lenck S, Degos V, Sourour NA, Frasca Polara G, Shotar E, et al. Cangrelor and stenting in acute ischemic stroke: monocentric case series. Clin Neuroradiol. 2021; 31:439–448.
72. Bang OY. Intracranial atherosclerosis: current understanding and perspectives. J Stroke. 2014; 16:27–35.

73. Park H, Baek JH, Kim BM. Endovascular treatment of acute stroke due to intracranial atherosclerotic stenosis-related large vessel occlusion. Front Neurol. 2019; 10:308.

74. Baek JH, Kim BM, Heo JH, Kim DJ, Nam HS, Kim YD, et al. Association between flat-panel computed tomography hyperattenuation and clinical outcome after successful recanalization by endovascular treatment. J Neurosurg. 2021; 135:704–711.

75. Mori T, Fukuoka M, Kazita K, Mori K. Follow-up study after intracranial percutaneous transluminal cerebral balloon angioplasty. AJNR Am J Neuroradiol. 1998; 19:1525–1533.

76. Miao Z, Song L, Liebeskind DS, Liu L, Ma N, Wang Y, et al. Outcomes of tailored angioplasty and/or stenting for symptomatic intracranial atherosclerosis: a prospective cohort study after SAMMPRIS. J Neurointerv Surg. 2015; 7:331–335.

77. Yaghi S, Khatri P, de Havenon A, Yeatts S, Chang AD, Cutting S, et al. Peri-procedural stroke or death in stenting of symptomatic severe intracranial stenosis. J Neurointerv Surg. 2020; 12:374–379.

78. Yu SC, Leung TW, Lee KT, Wong LK. Angioplasty and stenting of intracranial atherosclerosis with the Wingspan system: 1-year clinical and radiological outcome in a single Asian center. J Neurointerv Surg. 2014; 6:96–102.

79. Vajda Z, Schmid E, Güthe T, Klötzsch C, Lindner A, Niehaus L, et al. The modified Bose method for the endovascular treatment of intracranial atherosclerotic arterial stenoses using the Enterprise stent. Neurosurgery. 2012; 70:91–101.

80. Alexander MJ, Zauner A, Chaloupka JC, Baxter B, Callison RC, Gupta R, et al. WEAVE trial: final results in 152 on-label patients. Stroke. 2019; 50:889–894.
81. Stracke CP, Fiehler J, Meyer L, Thomalla G, Krause LU, Lowens S, et al. Emergency intracranial stenting in acute stroke: predictors for poor outcome and for complications. J Am Heart Assoc. 2020; 9:e012795.

82. Baek JH, Kim BM, Kim DJ, Heo JH, Nam HS, Yoo J. Stenting as a rescue treatment after failure of mechanical thrombectomy for anterior circulation large artery occlusion. Stroke. 2016; 47:2360–2363.

83. Chang Y, Kim BM, Bang OY, Baek JH, Heo JH, Nam HS, et al. Rescue stenting for failed mechanical thrombectomy in acute ischemic stroke: a multicenter experience. Stroke. 2018; 49:958–964.
84. Baracchini C, Farina F, Soso M, Viaro F, Favaretto S, Palmieri A, et al. Stentriever thrombectomy failure: a challenge in stroke management. World Neurosurg. 2017; 103:57–64.

85. Cornelissen SA, Andersson T, Holmberg A, Brouwer PA, Söderman M, Bhogal P, et al. Intracranial stenting after failure of thrombectomy with the emboTrap ® device. Clin Neuroradiol. 2019; 29:677–683.
86. Peng F, Wan J, Liu W, Huang W, Wang L, Qiu T, et al. Efficacy and safety of rescue stenting following failed mechanical thrombectomy for anterior circulation large vessel occlusion: propensity score analysis. J Neurointerv Surg. 2020; 12:271–273.

87. Premat K, Dechartres A, Lenck S, Shotar E, Le Bouc R, Degos V, et al. Rescue stenting versus medical care alone in refractory large vessel occlusions: a systematic review and meta-analysis. Neuroradiology. 2020; 62:629–637.

88. Duan G, Feng Z, Zhang L, Zhang P, Chen L, Hong B, et al. Solitaire stents for the treatment of complex symptomatic intracranial stenosis after antithrombotic failure: safety and efficacy evaluation. J Neurointerv Surg. 2016; 8:680–684.

89. Lee KY, Chen DY, Hsu HL, Chen CJ, Tseng YC. Undersized angioplasty and stenting of symptomatic intracranial tight stenosis with Enterprise: evaluation of clinical and vascular outcome. Interv Neuroradiol. 2016; 22:187–195.

90. Huang CM, Hong YF, Xing SH, Xu K, Xu CK, Zhang WJ, et al. Thirty-day outcomes of the enterprise stent in treating hypoperfusion of symptomatic intracranial stenosis. World Neurosurg. 2019; 129:e429–e435.

91. Salik AE, Selcuk HH, Zalov H, Kilinc F, Cirak M, Kara B. Medium-term results of undersized angioplasty and stenting for symptomatic high-grade intracranial atherosclerotic stenosis with Enterprise. Interv Neuroradiol. 2019; 25:484–490.

92. Feng Z, Duan G, Zhang P, Chen L, Xu Y, Hong B, et al. Enterprise stent for the treatment of symptomatic intracranial atherosclerotic stenosis: an initial experience of 44 patients. BMC Neurol. 2015; 15:187.

93. Vajda Z, Güthe T, Perez MA, Kurre W, Schmid E, Bäzner H, et al. Prevention of intracranial in-stent restenoses: predilatation with a drug eluting balloon, followed by the deployment of a self-expanding stent. Cardiovasc Intervent Radiol. 2013; 36:346–352.

94. Xu H, Quan T, Zaidat OO, Chen D, Wang Z, Yuan Y, et al. Neuroform EZ stenting for symptomatic intracranial artery stenosis: 30 days outcomes in a high-volume stroke center. Front Neurol. 2019; 10:428.

95. Sweid A, Herial N, Sajja K, Chalouhi N, Velagapudi L, Doermann A, et al. Early multicenter experience with the Neuroform Atlas stent: feasibility, safety, and efficacy. Neurosurgery. 2020; 87:E321–E335.

96. Miao Z, Zhang Y, Shuai J, Jiang C, Zhu Q, Chen K, et al. Thirty-day outcome of a multicenter registry study of stenting for symptomatic intracranial artery stenosis in China. Stroke. 2015; 46:2822–2829.

97. Hassan AE, Mohammaden MH, Rabah RR, Tekle WG. Initial experience with the next-generation resolute onyx zotarolimus-eluting stent in symptomatic intracranial atherosclerotic disease. Front Neurol. 2020; 11:570100.

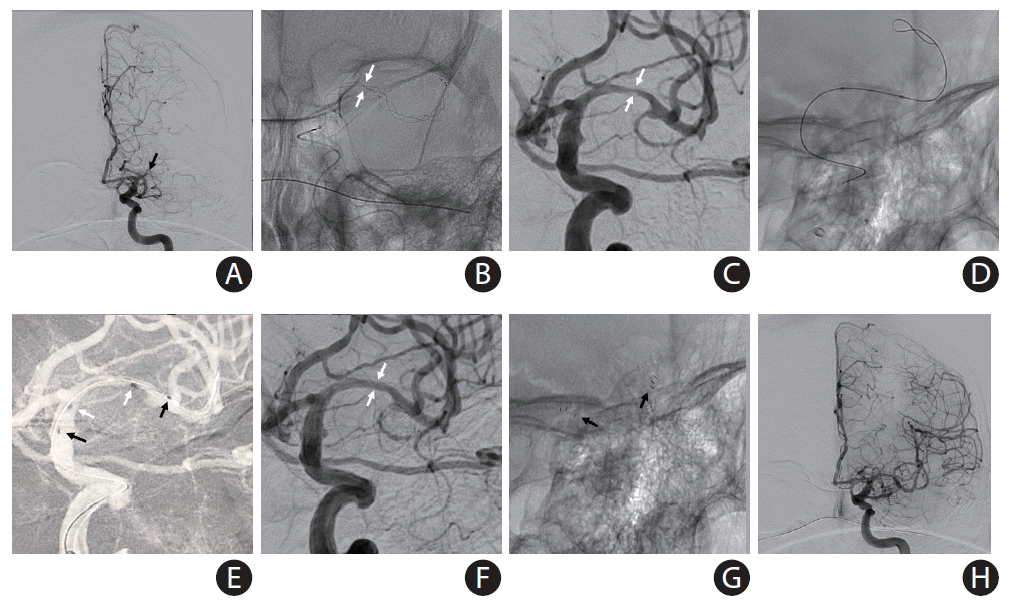
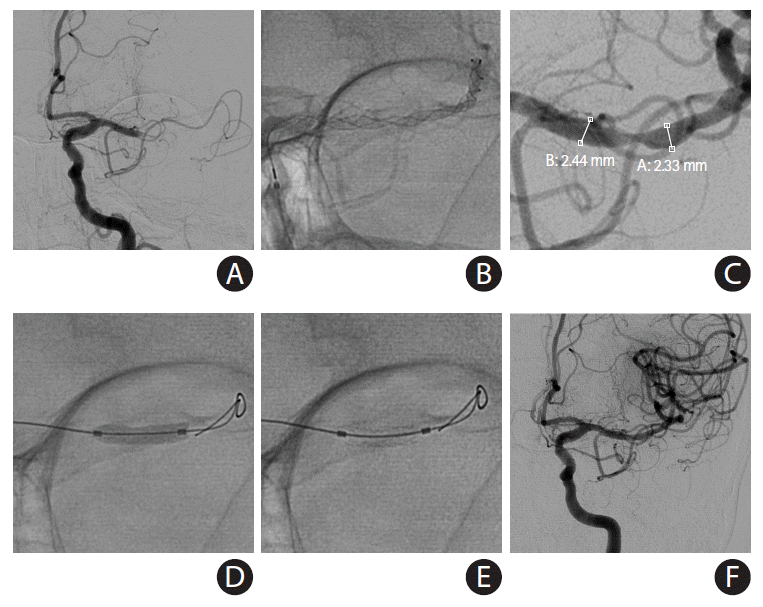
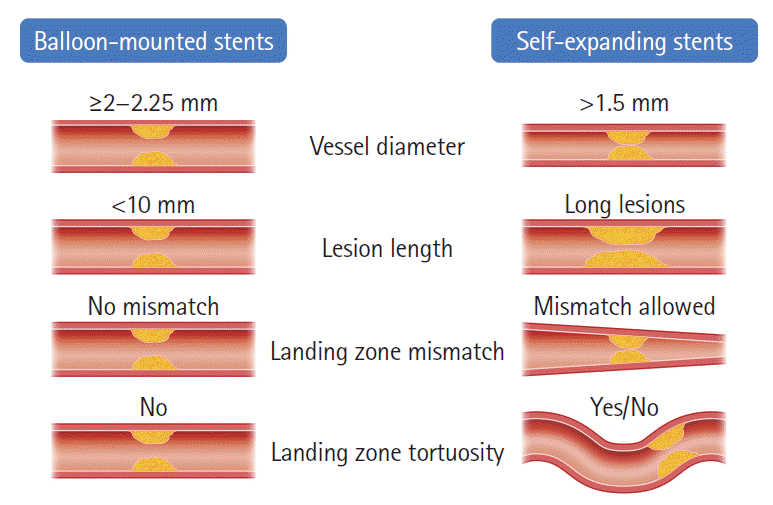




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download