1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020; 382:727–733.

3. Mafham MM, Spata E, Goldacre R, Gair D, Curnow P, Bray M, et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020; 396:381–389.

4. Schwarz V, Mahfoud F, Lauder L, Reith W, Behnke S, Smola S, et al. Decline of emergency admissions for cardiovascular and cerebrovascular events after the outbreak of COVID-19. Clin Res Cardiol. 2020; 109:1500–1506.

5. Butt AA, Kartha AB, Masoodi NA, Azad AM, Asaad NA, Alhomsi MU, et al. Hospital admission rates, length of stay, and in-hospital mortality for common acute care conditions in COVID-19 vs. pre-COVID-19 era. Public Health. 2020; 189:6–11.

6. Kuitunen I, Ponkilainen VT, Launonen AP, Reito A, Hevonkorpi TP, Paloneva J, et al. The effect of national lockdown due to COVID-19 on emergency department visits. Scand J Trauma Resusc Emerg Med. 2020; 28:114.

7. Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 2020; 382:e60.

8. Akhtar N, Al Jerdi S, Mahfoud Z, Imam Y, Kamran S, Saqqur M, et al. Impact of COVID-19 pandemic on stroke admissions in Qatar. BMJ Neurol Open. 2021; 3:e000084.

9. Mehrpour M, Shuaib A, Farahani M, Hatamabadi HR, Fatehi Z, Ghaffari M, et al. Coronavirus disease 2019 and stroke in Iran: a case series and effects on stroke admissions. Int J Stroke. 2020; Jun. 26. [Epub].
https://doi.org/10.1177/1747493020937397.

10. Aguiar de Sousa D, Sandset EC, Elkind MSV. The curious case of the missing strokes during the COVID-19 pandemic. Stroke. 2020; 51:1921–1923.

11. Padmanabhan N, Natarajan I, Gunston R, Raseta M, Roffe C. Impact of COVID-19 on stroke admissions, treatments, and outcomes at a comprehensive stroke centre in the United Kingdom. Neurol Sci. 2021; 42:15–20.

12. Uchino K, Kolikonda MK, Brown D, Kovi S, Collins D, Khawaja Z, et al. Decline in stroke presentations during COVID-19 surge. Stroke. 2020; 51:2544–2547.

13. Perry R, Banaras A, Werring DJ, Simister R. What has caused the fall in stroke admissions during the COVID-19 pandemic? J Neurol. 2020; 267:3457–3458.

14. Rudilosso S, Laredo C, Vera V, Vargas M, Renú A, Llull L, et al. Acute stroke care is at risk in the era of COVID-19: experience at a comprehensive stroke center in Barcelona. Stroke. 2020; 51:1991–1995.

15. D’Anna L, Sheikh A, Bathula R, Elmamoun S, Oppong A, Singh R, et al. Decreasing referrals to transient ischaemic attack clinics during the COVID-19 outbreak: results from a multicentre cross-sectional survey. BMJ Open. 2020; 10:e041514.

16. Butt JH, Fosbøl EL, Østergaard L, Yafasova A, Andersson C, Schou M, et al. Effect of COVID-19 on first-time acute stroke and transient ischemic attack admission rates and prognosis in Denmark: a nationwide cohort study. Circulation. 2020; 142:1227–1229.
17. Kansagra AP, Goyal MS, Hamilton S, Albers GW. Collateral effect of COVID-19 on stroke evaluation in the United States. N Engl J Med. 2020; 383:400–401.

18. Bres Bullrich M, Fridman S, Mandzia JL, Mai LM, Khaw A, Vargas Gonzalez JC, et al. COVID-19: stroke admissions, emergency department visits, and prevention clinic referrals. Can J Neurol Sci. 2020; 47:693–696.

19. Kim YD, Nam HS, Sohn SI, Park H, Hong JH, Kim GS, et al. Care process of recanalization therapy for acute stroke during the COVID-19 outbreak in South Korea. J Clin Neurol. 2021; 17:63–69.

20. Pasarikovski CR, da Costa L. The impact of the COVID-19 pandemic on stroke volume. Can J Neurol Sci. 2020; 47:847–848.

21. Diegoli H, Magalhães PS, Martins SC, Moro CH, França PH, Safanelli J, et al. Decrease in hospital admissions for transient ischemic attack, mild, and moderate stroke during the COVID-19 era. Stroke. 2020; 51:2315–2321.

22. Schlachetzki F, Wilfling S, Hubert ND, Wagner A, Haberl RL, Linker RA, et al. Decline and recurrence of stroke consultations during the COVID-19 pandemic lockdown parallels population activity levels. Cerebrovasc Dis. 2021; 50:317–325.

23. Kato A, Minami Y, Katsura A, Muramatsu Y, Sato T, Kakizaki R, et al. Physical exertion as a trigger of acute coronary syndrome caused by plaque erosion. J Thromb Thrombolysis. 2020; 49:377–385.

24. Grau AJ, Urbanek C, Palm F. Common infections and the risk of stroke. Nat Rev Neurol. 2010; 6:681–694.

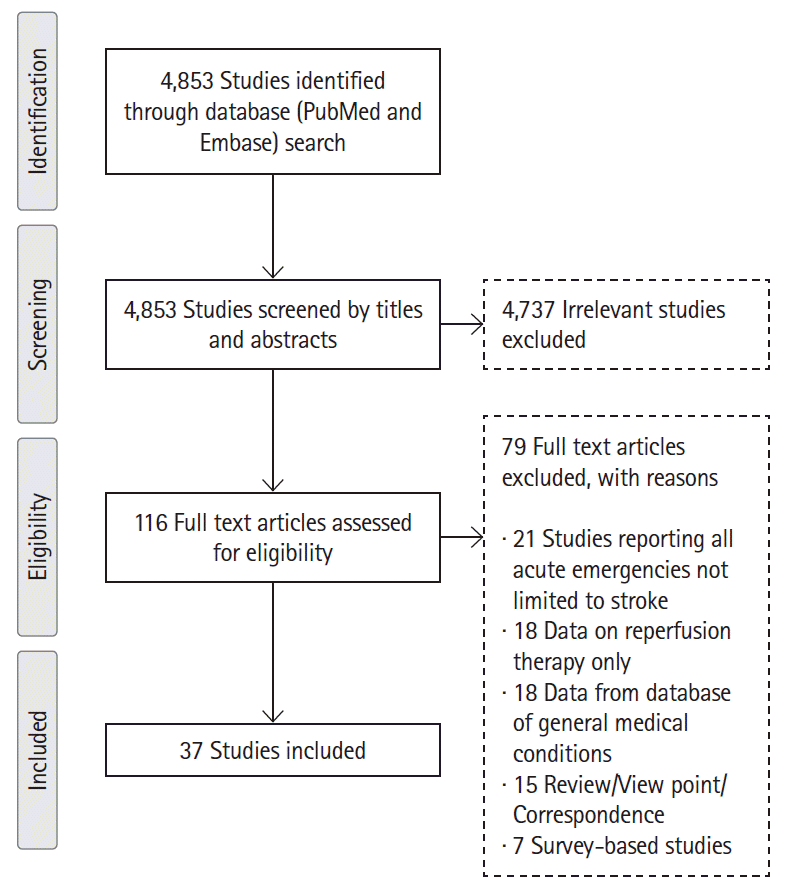
25. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372:n71.
26. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. Ottawa Hospital Research Institute. Ottawa Hospital Research Institute;
http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. 2021. Accessed October 30, 2021.
27. McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A; DEPRESsion Screening Data (DEPRESSD) Collaboration. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. 2020; Jan. 30. [Epub].
https://doi.org/10.1177/0962280219889080.

28. Balestrino M, Coccia A, Boffa AS, Furgani A, Bermano F, Finocchi C, et al. Request of hospital care dropped for TIA but remained stable for stroke during COVID-19 pandemic at a large Italian university hospital. Intern Emerg Med. 2021; 16:735–739.

29. Balucani C, Carhuapoma JR, Canner JK, Faigle R, Johnson B, Aycock A, et al. Exploring the collateral damage of the COVID-19 pandemic on stroke care: a statewide analysis. Stroke. 2021; 52:1822–1825.

30. Brunetti V, Broccolini A, Caliandro P, Di Iorio R, Monforte M, Morosetti R, et al. Effect of the COVID-19 pandemic and the lockdown measures on the local stroke network. Neurol Sci. 2021; 42:1237–1245.

31. Desai SM, Guyette FX, Martin-Gill C, Jadhav AP. Collateral damage: impact of a pandemic on stroke emergency services. J Stroke Cerebrovasc Dis. 2020; 29:104988.
32. Esenwa C, Parides MK, Labovitz DL. The effect of COVID-19 on stroke hospitalizations in New York City. J Stroke Cerebrovasc Dis. 2020; 29:105114.

33. Gdovinová Z, Vitková M, Baráková A, Cvopová A. The impact of the COVID-19 outbreak on acute stroke care in Slovakia: data from across the country. Eur J Neurol. 2021; 28:3263–3266.

34. Hasan AT, Das SC, Islam MS, Mansur M, Shawon MS, Hassan R, et al. Impact of COVID-19 on hospital admission of acute stroke patients in Bangladesh. PLoS One. 2021; 16:e0240385.

35. de Havenon A, Ney J, Callaghan B, Delic A, Hohmann S, Shippey E, et al. A rapid decrease in stroke, acute coronary syndrome, and corresponding interventions at 65 United States hospitals following emergence of COVID-19. medRxiv. 2020; May. 11.
https://doi.org/10.1101/2020.05.07.20083386.

36. Kim TJ, Kim BJ, Gwak DS, Lee JS, Kim JY, Lee KJ, et al. Modification of acute stroke pathway in Korea after the coronavirus disease 2019 outbreak. Front Neurol. 2020; 11:597785.

37. Kristoffersen ES, Jahr SH, Thommessen B, Rønning OM. Effect of COVID-19 pandemic on stroke admission rates in a Norwegian population. Acta Neurol Scand. 2020; 142:632–636.

38. Kwan J, Brown M, Bentley P, Brown Z, D’Anna L, Hall C, et al. Impact of COVID-19 pandemic on a regional stroke thrombectomy service in the United Kingdom. Cerebrovasc Dis. 2021; 50:178–184.

39. Ramírez-Moreno JM, Portilla-Cuenca JC, Hariramani-Ramchandani R, Rebollo B, Bermejo Casado I, Macías-Sedas P, et al. Slump in hospital admissions for stroke, a fact of an uncertain nature that requires explanation. Brain Sci. 2021; 11:92.

40. Nogueira RG, Qureshi MM, Abdalkader M, Martins SO, Yamagami H, Qiu Z, et al. Global impact of COVID-19 on stroke care and IV thrombolysis. Neurology. 2021; 96:e2824–e2838.
41. Ortega-Gutierrez S, Farooqui M, Zha A, Czap A, Sebaugh J, Desai S, et al. Decline in mild stroke presentations and intravenous thrombolysis during the COVID-19 pandemic: the Society of Vascular and Interventional Neurology Multicenter Collaboration. Clin Neurol Neurosurg. 2021; 201:106436.
42. Pandey AS, Daou BJ, Tsai JP, Zaidi SF, Salahuddin H, Gemmete JJ, et al. Letter: COVID-19 pandemic: the Bystander effect on stroke care in Michigan. Neurosurgery. 2020; 87:E397–E399.
43. Richter D, Eyding J, Weber R, Bartig D, Grau A, Hacke W, et al. Analysis of nationwide stroke patient care in times of COVID-19 pandemic in Germany. Stroke. 2021; 52:716–721.

44. Rinkel LA, Prick JC, Slot RE, Sombroek NM, Burggraaff J, Groot AE, et al. Impact of the COVID-19 outbreak on acute stroke care. J Neurol. 2021; 268:403–408.

45. Sacco S, Ricci S, Ornello R, Eusebi P, Petraglia L, Toni D, et al. Reduced admissions for cerebrovascular events during COVID-19 outbreak in Italy. Stroke. 2020; 51:3746–3750.
46. Sharma M, Lioutas VA, Madsen T, Clark J, O’Sullivan J, Elkind MS, et al. Decline in stroke alerts and hospitalisations during the COVID-19 pandemic. Stroke Vasc Neurol. 2020; 5:403–405.

47. Słowik A, Nowak R, Popiela T. Significant fall in stroke admissions in the Malopolska Voivodeship of Poland during the COVID-19 pandemic. Neurol Neurochir Pol. 2020; 54:471–472.

48. Tavanaei R, Yazdani KO, Akhlaghpasand M, Zali A, OraeeYazdani S. Changed pattern of hospital admission in stroke during COVID-19 pandemic period in Iran: a retrospective study. Neurol Sci. 2021; 42:445–453.

49. Mag Uidhir F, Bathula R, Sivagnanaratnam A, Abdul-Saheb M, Devine J, Cohen DL. Impact of COVID-19 on stroke caseload in a major hyperacute stroke unit. J Stroke Cerebrovasc Dis. 2020; 29:105383.
50. Uphaus T, Gröschel S, Hayani E, Hahn M, Steffen F, Gröschel K. Stroke care within the COVID-19 pandemic-increasing awareness of transient and mild stroke symptoms needed. Front Neurol. 2020; 11:581394.

51. Wang J, Chaudhry SA, Tahsili-Fahadan P, Altaweel LR, Bashir S, Bahiru Z, et al. The impact of COVID-19 on acute ischemic stroke admissions: analysis from a community-based tertiary care center. J Stroke Cerebrovasc Dis. 2020; 29:105344.

52. Wu Y, Chen F, Wang Z, Feng W, Liu Y, Wang Y, et al. Reductions in hospital admissions and delays in acute stroke care during the pandemic of COVID-19. Front Neurol. 2020; 11:584734.

53. Zhang LL, Guo YJ, Lin YP, Hu RZ, Yu JP, Yang J, et al. Stroke care in the first affiliated hospital of Chengdu Medical College during the COVID-19 outbreak. Eur Neurol. 2020; 83:630–635.

54. Aboul Nour H, Affan M, Mohamed G, Mohamud A, Schultz L, Latack K, et al. Impact of the COVID-19 pandemic on acute stroke care, time metrics, outcomes, and racial disparities in a Southeast Michigan Health System. J Stroke Cerebrovasc Dis. 2021; 30:105746.

55. D’Anna L, Brown M, Oishi S, Ellis N, Brown Z, Bentley P, et al. Impact of national lockdown on the hyperacute stroke care and rapid transient ischaemic attack outpatient service in a comprehensive tertiary stroke centre during the COVID-19 pandemic. Front Neurol. 2021; 12:627493.

56. Dębiec A, Bilik M, Piasecki P, Stępień A, Staszewski J. Effect of COVID-19 pandemic on stroke admissions and quality of stroke interventional treatment in Masovian Voivodeship. Neurol Neurochir Pol. 2021; 55:223–226.

57. Douiri A, Muruet W, Bhalla A, James M, Paley L, Stanley K, et al. Stroke care in the United Kingdom during the COVID-19 pandemic. Stroke. 2021; 52:2125–2133.

58. Jansen R, Lee JI, Turowski B, Kaschner M, Caspers J, Bernhard M, et al. Consequences of COVID-19 pandemic lockdown on emergency and stroke care in a German tertiary stroke center. Neurol Res Pract. 2021; 3:21.

59. Libruder C, Ram A, Hershkovitz Y, Tanne D, Bornstein NM, Leker RR, et al. Reduction in acute stroke admissions during the COVID-19 pandemic: data from a National Stroke Registry. Neuroepidemiology. 2021; 55:354–360.

60. Mariet AS, Giroud M, Benzenine E, Cottenet J, Roussot A, Aho-Glélé LS, et al. Hospitalizations for stroke in France during the COVID-19 pandemic before, during, and after the national lockdown. Stroke. 2021; 52:1362–1369.

61. Melaika K, Sveikata L, Wiśniewski A, Jaxybayeva A, Ekkert A, Jatužis D, et al. Changes in prehospital stroke care and stroke mimic patterns during the COVID-19 lockdown. Int J Environ Res Public Health. 2021; 18:2150.

62. Raymaekers V, Demeestere J, Bellante F, De Blauwe S, De Raedt S, Dusart A, et al. The impact of COVID-19 on acute stroke care in Belgium. Acta Neurol Belg. 2021; 121:1251–1258.

63. Wallace AN, Asif KS, Sahlein DH, Warach SJ, Malisch T, LaFranchise EF, et al. Patient characteristics and outcomes associated with decline in stroke volumes during the early COVID-19 pandemic. J Stroke Cerebrovasc Dis. 2021; 30:105569.

64. Katsanos AH, Palaiodimou L, Zand R, Yaghi S, Kamel H, Navi BB, et al. The impact of SARS-CoV-2 on stroke epidemiology and care: a meta-analysis. Ann Neurol. 2021; 89:380–388.
65. Nannoni S, de Groot R, Bell S, Markus HS. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke. 2021; 16:137–149.

66. Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, et al. COVID-19 and cardiovascular disease. Circulation. 2020; 141:1648–1655.

67. Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020; 191:9–14.

68. Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020; 19:919–929.

69. Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020; 173:268–277.
70. Teo KC, Leung WC, Wong YK, Liu RK, Chan AH, Choi OM, et al. Delays in stroke onset to hospital arrival time during COVID-19. Stroke. 2020; 51:2228–2231.

71. Meyer D, Meyer BC, Rapp KS, Modir R, Agrawal K, Hailey L, et al. A stroke care model at an academic, comprehensive stroke center during the 2020 COVID-19 pandemic. J Stroke Cerebrovasc Dis. 2020; 29:104927.

72. Singh S, Fong HK, Desai R, Zwinderman AH. Impact of COVID-19 on acute coronary syndrome-related hospitalizations: a pooled analysis. Int J Cardiol Heart Vasc. 2021; 32:100718.

73. Blecker S, Jones SA, Petrilli CM, Admon AJ, Weerahandi H, Francois F, et al. Hospitalizations for chronic disease and acute conditions in the time of COVID-19. JAMA Intern Med. 2021; 181:269–271.

75. Nguyen TN, Haussen DC, Qureshi MM, Yamagami H, Fujinaka T, Mansour OY, et al. Decline in subarachnoid haemorrhage volumes associated with the first wave of the COVID-19 pandemic. Stroke Vasc Neurol. 2021; Mar. 26. [Epub].
https://doi.org/10.1136/svn-2020-000695.

76. Tsigkas G, Koufou EE, Katsanos K, Patrinos P, Moulias A, Miliordos I, et al. Potential relationship between lifestyle changes and incidence of hospital admissions for acute coronary syndrome during the COVID-19 lockdown. Front Cardiovasc Med. 2021; 8:604374.

77. Versaci F, Gaspardone A, Danesi A, Ferranti F, Mancone M, Mariano E, et al. Interplay between COVID-19, pollution, and weather features on changes in the incidence of acute coronary syndromes in early 2020. Int J Cardiol. 2021; 329:251–259.

78. Qian Z, Kang H, Tang K, Jiang C, Wu Z, Li Y, et al. Assessment of risk of aneurysmal rupture in patients with normotensives, controlled hypertension, and uncontrolled hypertension. J Stroke Cerebrovasc Dis. 2016; 25:1746–1752.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download