Abstract
In mechanically ventilated patients, assisted mechanical ventilation (MV) is employed early, following the acute phase of critical illness, in order to eliminate the detrimental effects of controlled MV, most notably the development of ventilator-induced diaphragmatic dysfunction. Nevertheless, the benefits of assisted MV are often counteracted by the development of patient-ventilator dyssynchrony. Patient-ventilator dyssynchrony occurs when either the initiation and/or termination of mechanical breath is not in time agreement with the initiation and termination of neural inspiration, respectively, or if the magnitude of mechanical assist does not respond to the patient’s respiratory demand. As patient-ventilator dyssynchrony has been associated with several adverse effects and can adversely influence patient outcome, every effort should be made to recognize and correct this occurrence at bedside. To detect patient-ventilator dyssynchronies, the physician should assess patient comfort and carefully inspect the pressure- and flow-time waveforms, available on the ventilator screen of all modern ventilators. Modern ventilators offer several modifiable settings to improve patient-ventilator interaction. New proportional modes of ventilation are also very helpful in improving patient-ventilator interaction.
Mechanical ventilation (MV) can be distinguished in two major categories: controlled MV, where the act of breathing is entirely controlled by the ventilator and assisted MV, where the patients’ respiratory system and the ventilator work together. Controlled ventilation is mainly applied for short periods, especially during the acute phase of illness. As soon as there are no contraindications to sedation reduction and the patient is able to breathe spontaneously, an assisted ventilator mode is applied. This practice has been dictated by a plethora of evidence emphasizing the detrimental effects of controlled MV and associated respiratory muscles inactivation, most notably the development of ventilator-induced diaphragmatic dysfunction [1,2]. Contrariwise, assisted ventilation is associated with reduced dose and duration of sedation and decreased used of neuromuscular blockade which have been shown to promote hemodynamic stability, diminish the risk of critical illness polyneuromyopathy, improve gas exchange and shorten the duration of MV [3-6].
With the onset of assisted MV, two entirely different systems are called to collaborate: the respiratory system and the ventilator. The quality of their interaction, temporal and quantitative, is imperative for MV to be beneficial and is described by the Greek word “synchrony.” Patient-ventilator synchrony occurs when (1) the ventilator provides flow and pressure as soon as patient effort begins; (2) the magnitude of this pressure and flow meets patient respiratory demand; and (3) the ventilator assistance is terminated when patient effort ends. Whenever any of the above is not fulfilled, patient-ventilator dyssynchrony occurs. Adverse effects of patient-ventilator dyssynchrony include: increased work of breathing, patient discomfort, alveolar overdistention and lung injury, sleep disturbances, periodic breathing, unnecessary use of sedation and excessive unloading of the diaphragm leading to ventilator-induced diaphragmatic dysfunction [7-10]. To detect patient-ventilator dyssynchronies, the physician should assess patient comfort and carefully inspect the pressure- and flow-time waveforms, available on the ventilator screen of all modern ventilators.
This review aims to describe the various forms of patient-ventilator dyssynchrony, focusing on how to identify them at the bedside, to analyze their causes, the actions required to reduce or eliminate them and their clinical impact. Before that, a brief revision on the pathophysiology of breathing and the basic principles dictating the operation of assisted ventilatory modes will be provided.
To understand patient-ventilator dyssynchrony during assisted MV, one must first understand the pressures developed in the respiratory system during a spontaneous breath. To initiate a breath, the respiratory muscles contract, obeying nerve stimuli coming from the respiratory control center. Inspiratory muscle contraction expands the alveoli and decreases alveolar pressure below atmospheric pressure, driving gas into the lungs. Respiratory muscle contraction generates a pressure (Pmus), which at any time during inspiration (t), is dissipated to overcome two pressures opposing respiratory system inflation, the resistive (Pres) and elastic (Pel) pressure of the respiratory system (inertia is assumed to be negligible) [11]. This is accurately described by the equation of motion of the respiratory system:
Where Rrs and Ers are resistance and elastance of the respiratory system respectively, ΔVFRC(t) is instantaneous volume above the passive functional residual capacity (FRC) and V’(t) is instantaneous flow. If ΔV is related to end-expiratory lung volume (EE),
where PEEPi, is the elastic recoil pressure of the respiratory system at end-expiration.
During control MV, the respiratory muscles do not contract, Pmus is 0 and the pressure needed to overcome the elastance and resistance of the respiratory system is entirely provided by the ventilator. The equation of motion in this case is modified as follows:
where Paw is the pressure provided by the ventilator.
In assisted MV, the total pressure applied to the respiratory systems comes both from the ventilator and the respiratory muscles and the equation of motion is modified as follows:
To interpret the basic waveforms and subsequently to recognize patient-ventilator dyssynchronies, it is useful to discuss the basic principles dictating the operation of assisted ventilator modes.
Three variables determine the function of a positive pressure ventilator: (1) the triggering variable, (2) the variable that controls the delivered pressure or flow during the mechanical inspiration, and (3) the cycling off variable.
The triggering variable is the signal that initiates the mechanical breath. In assisted ventilation, the most commonly used triggering variables are flow and pressure. Mechanical inspiration starts when patient inspiratory effort decreases either the flow (flow triggering) or the pressure (pressure triggering) in the ventilator circuit to a preset level. It is generally believed that flow triggering is more friendly to the patient regarding the work of breathing, because the pressure triggering necessitates isometric contraction of the respiratory muscles [12,13]. However, studies have shown that, in the modern ventilators, there are minimal differences, if any, concerning the work of breathing between the two ways of triggering [12-14]. Other triggering variables include flow waveform, volume, transdiaphragmatic pressure (Pdi)-driven servoventilation and the electromyographic activity of the diaphragm (EAdi; neurally adjusted ventilator assist [NAVA]) but their use in clinical practice is limited [12,15-21].
The variable that controls the pressure and flow delivery distinguishes the various assisted MV modes [22,23]. In assist volume control, the ventilator delivers a preset tidal volume with a preset flow-time profile. These are the independent variables, while the airway pressure (Paw) needed to deliver the preset flow and volume depends on the mechanical properties of the respiratory system and is the dependent variable. In pressure control or pressure support, the ventilator delivers a preset pressure (independent variable). The dependent variables on pressure-preset modes are the volume and flow. These variables change according to the mechanical properties of the respiratory system and the pressure delivered. In proportional modes of ventilation, no variable is preset: the ventilator delivers support which is proportional to patient inspiratory effort. The later is expressed either through changes in instantaneous flow and volume (proportional assist ventilation [PAV]), or through changes in the neural activity of the diaphragm (NAVA) [11,22,24].
The cycling off variable is defined as the signal of terminating the delivery phase. The usual cycling off criterion in assisted pressure-preset modes of MV is airway pressure or flow: the ventilator terminates assist and opens the expiratory valve when Paw increases or flow decreases to a preset criterion. Other cycling off criteria are time, volume, the flow-waveform method and the EAdi in NAVA [15]. Ideally, the cycling off should occur simultaneously with the end of neural inspiration. However, this fact rarely, if ever, happens and the expiratory asynchrony is a common event with all conventional modes of assisted ventilation.
With pressure-preset modes rise time is a setting that determines how fast the ventilator will reach the selected Paw. It is available in the new generation ventilators during pressure support and pressure control ventilation. Fast rising time is associated with a sharp increase in inspiratory flow and may cause pressure overshoot and promote dyspnoea. Very slow rising time may be recognized by a rounded shaped inspiratory flow [25-27]. Both the low and high rise time may alter the mechanical inspiratory time and the volume delivered and may provoke dissociation between mechanical and neural inspiration [27,28]. Since, there are no rules for setting an optimal rise time, both very rapid and slow rise time should be avoided and rising time should be adjusted according to patient respiratory drive [25-28].
Patient-ventilator dyssynchronies can be distinguished in two major categories: (1) dyssynchronies that occur because neural breath is not in phase with mechanical breath. This group includes: triggering delay, ineffective efforts, autotriggering, reverse triggering, delayed opening of the expiratory valve, premature opening of the expiratory valve, double triggering, and breath stacking and (2) dyssynchronies related to a discrepancy between the level of assist that the patient needs and the actual assist that the ventilator provides.
Triggering delay is the time interval between the initiation of the neural and mechanical inspiration [11,18]. If esophageal pressure (Pes) or EAdi monitoring is available, it is observed as the time elapsed between the reduction in Pes or increase in EAdi (start of neural inspiration) and the abrupt increase of flow or Paw (start of mechanical inspiration).
Ineffective efforts are patient’s efforts that fail to trigger the ventilator. Although usually described as a triggeringassociated dyssynchrony, ineffective efforts can happen at any time during the mechanical breath, during mechanical inspiration, expiration or at the transition between these two phases. The gold standard for their recognition is the simultaneous observation of the patient inspiratory activity. This is achieved either through recording of Pes or EAdi (Figure 2). Both methods are invasive and require the insertion of specified catheters: esophageal catheter, for Pes and NAVA catheter, for EAdi. In most cases, ineffective efforts can be identified non-invasively at the bedside, by observing the flow-time and Paw-time waveforms at the ventilator screen. Ineffective efforts cause distortions in the Paw and, more obviously, in the flow curve: an abrupt increase in inspiratory flow and a decrease in expiratory flow indicate the presence of ineffective efforts during mechanical inspiration and expiration, respectively (Figures 2 and 3) [11,18].
Triggering delay and ineffective efforts share common pathophysiological mechanisms. Their causes can be classified into two main categories: ventilator settings and patients’ characteristics [8,29-31]. Ventilator settings that predispose to triggering delay and ineffective efforts are the high assist level, the delayed opening of the expiratory valve and the low triggering sensitivity. These factors are determined by the physician. The main patients’ characteristics include low respiratory drive (i.e., sedation, central nervous diseases, etc.), weak inspiratory muscles (i.e., critical illness polyneuromyopathy, myasthenia, etc.), and high resistance and compliance that increase the time constant of the respiratory system.
The incidence and the magnitude of the delay of triggering and ineffective efforts are not minor in clinical practice. Ineffective efforts are the commonest form of patient-ventilator dyssynchrony. Vaporidi et al. [32] evaluated the role of ineffective efforts, specifically clusters of them, during MV on the outcome of critically ill patients. Events of ineffective efforts were identified in 38% of patients with prolonged MV and these events were associated with prolonged MV and increased mortality. Their incidence is higher in patients with obstructive lung disease [29,33]. In these patients, the low elastic recoil pressure and/or increased resistance increase the time required for the patient to exhale (high time constant), predisposing to air trapping and dynamic hyperinflation. Dynamic hyperinflation is the most important cause of triggering delay and ineffective efforts. In the presence of dynamic hyperinflation, an elastic threshold load (PEEPi) is imposed on the inspiratory muscles at the beginning of inspiration. The inspiratory muscles must first counterbalance PEEPi in order to decrease alveolar pressure below external positive end-expiratory pressure (PEEPe) and trigger the ventilator [34-36]. This creates a delay between the beginning of inspiratory effort and ventilator triggering. In severe cases of dynamic hyperinflation, especially if the effort is weak, the patient may fail to trigger the ventilator (ineffective effort). High airway resistance can be recognized in all ventilator modes by observing the expiratory flow-time curve. Early in expiration, a spike in expiratory flow signifies the dynamic compression of central airways [11]. Thereafter, the expiratory flow decreases but very slowly, if ever, returns to zero line before the following inspiration (Figures 2 and 3).
Delayed or missed triggers represent eccentric contractions of the diaphragm which may be deleterious for its function. They may cause distress and in their presence, the respiratory rate on the ventilator screen does not reflect the true respiratory rate of the patient. Strategies in order to reduce the triggering delay and the number of ineffective efforts should be investigated [8,11,22,29,31]. Firstly, ventilator settings should be revised. Setting the most sensitive triggering that does not cause autotriggering is an option. With regard to triggering sensitivity, it has been shown that the flow-waveform method [15], compared to flow triggering, enhances the sensitivity and thus reduces the incidence of these events. Furthermore, the newer methods for triggering in the context of Pdidriven servoventilation [20] and NAVA [21] eliminate these events. Ventilator settings that decrease dynamic hyperinflation and, hence, triggering delay and ineffective efforts are: (1) reduction of minute ventilation by lowering assist (decrease set pressure, set tidal volume) and respiratory rate decrease, (2) lengthening expiratory time through a higher flow threshold for cycling off and a faster rising time in pressure support or a higher inspiratory flow and a shorter plateau inspiratory pressure in assist volume control mode and (3) application of PEEPe. The PEEPe narrows the difference between the alveolar pressure and the threshold of pressure for the initiation of the mechanical inspiration, hence helping the patient to trigger the ventilator. This is especially helpful in some patients with severe obstructive lung disease [29,33]. Secondly, patient-related causes should be reviewed. Attention should be given in respiratory drive and the factors that may reduce it, such as excessive sedation and alkalemia. Efforts in order to decrease the magnitude of dynamic hyperinflation also should be done, in instance the use of corticosteroids, bronchodilating therapy and aspiration of secretions may result in reduced expiratory resistance.
Autotriggering occurs when the ventilator is triggered in the absence of patient effort [37,38]. This phenomenon can also be recognized by inspection of the ventilator waveforms. The absence of decrease in Pes (if it is monitored) or in Paw before the delivery phase, especially in the presence of a zero flow long enough before the mechanical breath, are signs that the breath is not triggered by the patient. The flow-time profile of the autotriggered breaths is, often, different from the corresponding of patient- triggered breaths (Figure 4). If secretions or cardiac oscillations are the cause, one might notice the associate flow distortion and suspect the phenomenon.
Autotriggering may be caused by a low threshold for triggering and/or artifacts that may cause a drop of Paw or flow, which is misinterpreted by the ventilator as patient effort. Artifacts that frequently cause autotriggering are circuit leaks, presence of water in the circuit, hiccups and strong cardiogenic oscillators [37,38]. Factors that predispose a patient to increased risk of autotriggering are the low respiratory drive and breathing frequency, the low time constant of the respiratory system (increased elastic recoil, low resistance) and the presence of hyperdynamic circulation (larger cardiac output and higher ventricular filling pressures).
Autotriggering is a common phenomenon during assisted modes of MV (invasive and non-invasive) [18,39]. When apparent, the respiratory rate is falsely elevated. These additional breaths may lead to hyperventilation, respiratory alkalosis, hyperinflation, and diaphragmatic dysfunction [2,40].
To eliminate autotriggering, the physician must try to correct the underlying cause: enhance the respiratory drive by reducing sedation, correct alkalosis by reducing the level of assist, aspirate secretions, and minimize circuit leaks. Moreover, a higher triggering threshold, or change from flow to pressure triggering may abolish autotriggered breaths [37,38].
When the exhalation valve of the ventilator opens too early, mechanical inspiration lasts shorter than neural inspiration. This form of dyssynchrony is often associated with insufficient support. Inspiratory muscle effort after the end of ventilator insufflation enhances the work of breathing and puts the patient at risk for double triggering. This asynchrony can present with the following ways [11]. (1) Zero or small inspiratory flow for some time after opening of the exhalation valve (Paw decreases to zero or PEEP level) indicates that inspiratory muscles continue to contract after the end of mechanical inspiration [41]. (2) A sharp decrease from the peak expiratory flow which lasts few milliseconds followed by an increase and then decreases gradually to zero toward the end of expiration: this pattern in the flow-time waveform is also a sign that considerable inspiratory muscle activity is present after opening of exhalation valve. When mechanical inspiration ends, end-inspiratory elastic recoil pressure is greater than inspiratory muscle pressure, creating positive alveolar pressure and, therefore, expiratory flow. Elastic recoil pressure decreases while lung volume declines due to inspiratory muscle contraction. An increasing opposing pressure to expiratory flow develops, causing an abrupt decrease in expiratory flow. This decrease is interrupted by the relaxation of the inspiratory muscles and expiratory flow increases and, therefore, follows the route as determined by the elastic recoil pressure and resistance of the patient and expiratory circuit [11]. (3) Double (or multiple) triggering: this refers to the delivery of two (or even more) ventilator insufflations during one single inspiratory effort. The inspiratory muscle contraction is great enough to overwhelm the elastic recoil pressure of the respiratory system and trigger the ventilator multiple times.
Premature cycling is associated with particular ventilator settings, such as low level of assist, relatively high threshold for cycling off and short inflation time. Short time constant of the respiratory system and long neural inspiration time are predisposing factors for this dyssynchrony. Furthermore, risk factors for multiple triggering are the low elastic recoil at the end of mechanical inflation and the intense inspiratory muscle activity [11,22].
As already mentioned, premature opening of the exhalation valve is related with increased work of breathing. Moreover, double triggering may provoke high tidal volumes (up to twice of the predetermined value) putting the patient at risk of ventilation-induced lung injury and ventilation-induced diaphragmatic dysfunction.
Premature opening of the expiratory valve and related multiple triggering can be minimized either by increasing the mechanical inspiration time and/or, if possible, by decreasing the neural inspiration time. The latter should be considered in patients with prolonged inspiratory efforts due to excessive administration of opioids. Actions that increase the mechanical inflation time include the lower flow threshold for cycling, the higher support and the slower rising time with pressure support mode [11,28]. With control modes, higher duration of mechanical inspiration should be easily achieved by proper adjustment of ventilator settings, such as inspiratory time, inspiratory flow or application of end-inspiratory pause [11,22].
When the exhalation valve opens too late, mechanical inspiration extends into neural expiration. This may promote rigorous expiratory muscle activity and increase the work of breathing as the patient struggles to terminate inspiration. Furthermore, it may unnecessarily increase the delivered tidal volume and shorten the available expiratory time. These effects may promote dynamic hyperinflation and associated dyssynchronies (delayed triggering, ineffective efforts), especially in patients with obstructive lung disease or provoke lung overdistention.
Recognition of the delayed opening of the exhalation valve is, often, challenging. During pressure support mode, it can be indicated by the fast decrease of the inspiratory flow followed by an exponential decline towards the end of mechanical inspiration [11]. An abrupt increase (spike) of the Paw near the end of mechanical inspiration is either a sign of inspiratory muscle relaxation or expiratory muscle contraction [42]. Whichever is the cause, this spike is an evidence of delayed cycling off (Figure 5). With assist-volume control ventilation, the sharp increase of Paw towards the end of mechanical breath indicates that mechanical inspiration is longer than neural.
Low flow threshold for cycling off, high support and low rise time are predisposing factors for this asynchrony during pressure support ventilation [43]. With assisted volume control mode, high tidal volume, increased inflation time, low inspiratory flow and application of end-inspiratory pause may causes delayed opening of the exhalation valve [43]. With pressure support mode, delayed opening of the expiratory valve is commonly observed in patients with long time constant of respiratory system, such as in patients with chronic obstructive lung diseases [44].
Independent of the mode of the ventilation, changes of the ventilator settings should be combined by measures that minimize the airway resistance and dynamic hyperinflation (i.e., bronchodilation, steroid therapy, aspiration of secretions).
Reverse triggering was first described by Akoumianaki et al. [45] who observed, in a group of heavily sedated
patients, the occurrence of patient inspiratory efforts that were triggered by the ventilator. This phenomenon, known as respiratory entrainment, has been previously described in animals, healthy subjects and preterm infants [40,46,47]. It differs from the other types of dyssynchronies in that the patient completely loses its normal breathing variability and actually breaths like a machine, exhibiting a fixed temporal relationship (1:1 or, less commonly, 1:2 or 1:3) between the onset of his inspiratory efforts and the onset of mechanical breaths. Vagal feedback and cortical influences are involved in the pathophysiological mechanism of these events [46,47]. Reverse triggered breaths may occur at any phase of the respiratory cycle and for variable periods. Their recognition, when Pes or EAdi recording is unavailable, is often difficult and requires careful inspection of the Paw- and flow-time waveforms (Figures 6 and 7). Depending on the phase of the mechanical breath that they occur, they may induce isometric or eccentric contractions of the respiratory muscles, augment the inflated tidal volume in pressure targeted modes or even trigger second breaths (breath stacking) causing hyperventilation and increasing the risk for lung overdistention (Figure 7) .
Inappropriate assist level (inadequate or excessive assist) with regard to patients’ ventilator demands is another cause of patient-ventilator dyssynchrony [11,22]. Inadequate assist level is usually observed in patients with high demands and increased respiratory drive (i.e., sepsis and metabolic acidosis). This discrepancy is combined with increased work of breathing and often the clinical status of the patient is indicative (i.e., use of accessory respiratory muscles, high respiratory rate).
Excessive assist level is often an outcome of low respiratory drive and/or inappropriate ventilator settings, but the adverse effects of this incompatibility are important. Dynamic hyperinflation, respiratory alkalosis, periodic breathing pattern due to lower apnoeic threshold, sleep disturbances, ventilator-induced diaphragmatic dysfunction and/or lung injury are potential consequences of high assist [44,48].
By inspecting the waveforms of Paw or flow, the respiratory effort in relation to assist level can be estimated [11,22]. Paw decreases when the inspiratory muscles contract and increases when the expiratory muscles contract. Paw is more sensitive in assist-volume control ventilation since it is the dependent variable. Although in pressure-targeted modes Paw should remain relatively constant, its shape may alter when the respiratory muscles contract rigorously (Figure 8). Furthermore, change in the flow pattern beyond the typical declining pattern is a sign of muscle effort [49]. Rapid decrease in inspiratory flow to flow threshold for cycling off in a patient with relatively long time constant is a sign of expiratory muscle contraction and thus a sign of high assist (Figure 9) [50]. Delayed cycling off might also indicate excessive assist. On the other hand, rounded or constant inspiratory flow represents significant inspiratory effort and might be due to insufficient assist (Figure 6). Supportive of insufficient assist is also the appearance of signs of premature cycling off or double triggering.
Apart from ventilator settings adjustments, the introduction of new modes of MV, the so-called proportional ventilation modes, was a major step towards a better patient-ventilator interaction. These modes share similar principles: they provide assistance in proportion to patient effort. Patient effort is expressed either as a change in instantaneous flow and volume (PAV) or as a change in the EAdi (NAVA). The clinician sets the “gain” to augment patient effort and pressure and flow delivery change breath by breath following patient ventilator demand. Triggering in PAV is similar to conventional assisted modes but in NAVA the EAdi triggers the ventilator. PAV measures also the respiratory mechanics through dedicated software. Several clinical studies have shown the PAV and NAVA greatly improve the synchrony between the patient and the ventilator. Specifically ineffective efforts, delayed or premature opening of the expiratory valve and excessive ventilator assist are greatly minimized [10,51-55]. The reason for improved patient-ventilator synchrony is the tight link between the patient’s effort and the ventilator support: the patient retains considerable control of his breathing pattern and of the tidal volume. Even at high levels of assist, negative feedbacks from the respiratory controller minimize the risk for excessive tidal volume, protecting from overdistention and overventilation [53,56].
Assisted MV offers significant advantages over controlled MV but attention should be paid on the presence of patient-ventilator dyssynchrony. Patient-ventilator dyssynchrony is common; it may occur at any phase of the respiratory cycle and can be due to time discrepancies or ventilator assist/respiratory demand discrepancies between the patient and the ventilator. It is associated with numerous unwanted effects and, may adversely affect the outcome. The gold standard for their recognition is the documentation of patient effort. However, in several cases, careful inspection of the ventilator screen along with the clinical picture of the patient is sufficient for the physician to recognize patient-ventilator dyssynchronies. Modern ventilators offer several modifiable settings to improve patient-ventilator interaction. New proportional modes of ventilation are also very helpful in improving patient-ventilator interaction.
References
1. Petrof BJ, Jaber S, Matecki S. Ventilator-induced diaphragmatic dysfunction. Curr Opin Crit Care. 2010; 16:19–25.

2. Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008; 358:1327–35.

3. Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000; 342:1471–7.

4. Schweickert WD, Gehlbach BK, Pohlman AS, Hall JB, Kress JP. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Med. 2004; 32:1272–6.

5. Garnacho-Montero J, Madrazo-Osuna J, Garcia- Garmendia JL, Ortiz-Leyba C, Jimenez-Jimenez FJ, Barrero-Almodovar A, et al. Critical illness polyneuropathy: risk factors and clinical consequences. A cohort study in septic patients. Intensive Care Med. 2001; 27:1288–96.

6. Putensen C, Zech S, Wrigge H, Zinserling J, Stuber F, Von Spiegel T, et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med. 2001; 164:43–9.

7. de Wit M, Miller KB, Green DA, Ostman HE, Gennings C, Epstein SK. Ineffective triggering predicts increased duration of mechanical ventilation. Crit Care Med. 2009; 37:2740–5.

8. Thille AW, Rodriguez P, Cabello B, Lellouche F, Brochard L. Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med. 2006; 32:1515–22.

9. Blanch L, Villagra A, Sales B, Montanya J, Lucangelo U, Lujan M, et al. Asynchronies during mechanical ventilation are associated with mortality. Intensive Care Med. 2015; 41:633–41.

10. Bosma K, Ferreyra G, Ambrogio C, Pasero D, Mirabella L, Braghiroli A, et al. Patient-ventilator interaction and sleep in mechanically ventilated patients: pressure support versus proportional assist ventilation. Crit Care Med. 2007; 35:1048–54.

11. Georgopoulos D, Prinianakis G, Kondili E. Bedside waveforms interpretation as a tool to identify patient-ventilator asynchronies. Intensive Care Med. 2006; 32:34–47.

12. Sassoon CS, Gruer SE. Characteristics of the ventilator pressure- and flow-trigger variables. Intensive Care Med. 1995; 21:159–68.
13. Aslanian P, El Atrous S, Isabey D, Valente E, Corsi D, Harf A, et al. Effects of flow triggering on breathing effort during partial ventilatory support. Am J Respir Crit Care Med. 1998; 157:135–43.

14. Goulet R, Hess D, Kacmarek RM. Pressure vs flow triggering during pressure support ventilation. Chest. 1997; 111:1649–53.

15. Prinianakis G, Kondili E, Georgopoulos D. Effects of the flow waveform method of triggering and cycling on patient-ventilator interaction during pressure support. Intensive Care Med. 2003; 29:1950–9.

16. Racca F, Squadrone V, Ranieri VM. Patient-ventilator interaction during the triggering phase. Respir Care Clin N Am. 2005; 11:225–45.

17. Slutsky AS. Mechanical ventilation: American College of Chest Physicians’ Consensus Conference. Chest. 1993; 104:1833–59.
18. Prinianakis G, Kondili E, Georgopoulos D. Patient-ventilator interaction: an overview. Respir Care Clin N Am. 2005; 11:201–24.

19. Tobin MJ, Jubran A, Laghi F. Patient-ventilator interaction. Am J Respir Crit Care Med. 2001; 163:1059–63.

20. Sharshar T, Desmarais G, Louis B, Macadou G, Porcher R, Harf A, et al. Transdiaphragmatic pressure control of airway pressure support in healthy subjects. Am J Respir Crit Care Med. 2003; 168:760–9.

21. Sinderby C, Navalesi P, Beck J, Skrobik Y, Comtois N, Friberg S, et al. Neural control of mechanical ventilation in respiratory failure. Nat Med. 1999; 5:1433–6.

22. Kondili E, Xirouchaki N, Georgopoulos D. Modulation and treatment of patient-ventilator dyssynchrony. Curr Opin Crit Care. 2007; 13:84–9.

23. Georgopoulos DB, Anastasaki M, Katsanoulas K. Effects of mechanical ventilation on control of breathing. Monaldi Arch Chest Dis. 1997; 52:253–62.
24. Brander L, Leong-Poi H, Beck J, Brunet F, Hutchison SJ, Slutsky AS, et al. Titration and implementation of neurally adjusted ventilatory assist in critically ill patients. Chest. 2009; 135:695–703.

25. Bonmarchand G, Chevron V, Chopin C, Jusserand D, Girault C, Moritz F, et al. Increased initial flow rate reduces inspiratory work of breathing during pressure support ventilation in patients with exacerbation of chronic obstructive pulmonary disease. Intensive Care Med. 1996; 22:1147–54.

26. Bonmarchand G, Chevron V, Ménard JF, Girault C, Moritz-Berthelot F, Pasquis P, et al. Effects of pressure ramp slope values on the work of breathing during pressure support ventilation in restrictive patients. Crit Care Med. 1999; 27:715–22.

27. Chiumello D, Pelosi P, Taccone P, Slutsky A, Gattinoni L. Effect of different inspiratory rise time and cycling off criteria during pressure support ventilation in patients recovering from acute lung injury. Crit Care Med. 2003; 31:2604–10.

28. Chiumello D, Pelosi P, Calvi E, Bigatello LM, Gattinoni L. Different modes of assisted ventilation in patients with acute respiratory failure. Eur Respir J. 2002; 20:925–33.

29. Thille AW, Cabello B, Galia F, Lyazidi A, Brochard L. Reduction of patient-ventilator asynchrony by reducing tidal volume during pressure-support ventilation. Intensive Care Med. 2008; 34:1477–86.

30. Leung P, Jubran A, Tobin MJ. Comparison of assisted ventilator modes on triggering, patient effort, and dyspnea. Am J Respir Crit Care Med. 1997; 155:1940–8.

31. Younes M, Kun J, Webster K, Roberts D. Response of ventilator-dependent patients to delayed opening of exhalation valve. Am J Respir Crit Care Med. 2002; 166:21–30.

32. Vaporidi K, Babalis D, Chytas A, Lilitsis E, Kondili E, Amargianitakis V, et al. Clusters of ineffective efforts during mechanical ventilation: impact on outcome. Intensive Care Med. 2017; 43:184–91.

33. Vitacca M, Bianchi L, Zanotti E, Vianello A, Barbano L, Porta R, et al. Assessment of physiologic variables and subjective comfort under different levels of pressure support ventilation. Chest. 2004; 126:851–9.

34. Nava S, Bruschi C, Rubini F, Palo A, Iotti G, Braschi A. Respiratory response and inspiratory effort during pressure support ventilation in COPD patients. Intensive Care Med. 1995; 21:871–9.

35. Rossi A, Polese G, Brandi G, Conti G. Intrinsic positive end-expiratory pressure (PEEPi). Intensive Care Med. 1995; 21:522–36.

36. Fabry B, Guttmann J, Eberhard L, Bauer T, Haberthür C, Wolff G. An analysis of desynchronization between the spontaneously breathing patient and ventilator during inspiratory pressure support. Chest. 1995; 107:1387–94.

37. Imanaka H, Nishimura M, Takeuchi M, Kimball WR, Yahagi N, Kumon K. Autotriggering caused by cardiogenic oscillation during flow-triggered mechanical ventilation. Crit Care Med. 2000; 28:402–7.

38. Hill LL, Pearl RG. Flow triggering, pressure triggering, and autotriggering during mechanical ventilation. Crit Care Med. 2000; 28:579–81.

39. Carteaux G, Lyazidi A, Cordoba-Izquierdo A, Vignaux L, Jolliet P, Thille AW, et al. Patient-ventilator asynchrony during noninvasive ventilation: a bench and clinical study. Chest. 2012; 142:367–76.
40. Sassoon CS, Zhu E, Caiozzo VJ. Assist-control mechanical ventilation attenuates ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med. 2004; 170:626–32.

42. Prinianakis G, Plataki M, Kondili E, Klimathianaki M, Vaporidi K, Georgopoulos D. Effects of relaxation of inspiratory muscles on ventilator pressure during pressure support. Intensive Care Med. 2008; 34:70–4.
43. Kondili E, Prinianakis G, Georgopoulos D. Patient-ventilator interaction. Br J Anaesth. 2003; 91:106–19.

44. Yamada Y, Du HL. Analysis of the mechanisms of expiratory asynchrony in pressure support ventilation: a mathematical approach. J Appl Physiol (1985). 2000; 88:2143–50.
45. Akoumianaki E, Lyazidi A, Rey N, Matamis D, Perez- Martinez N, Giraud R, et al. Mechanical ventilation-induced reverse-triggered breaths: a frequently unrecognized form of neuromechanical coupling. Chest. 2013; 143:927–38.
46. Simon PM, Habel AM, Daubenspeck JA, Leiter JC. Vagal feedback in the entrainment of respiration to mechanical ventilation in sleeping humans. J Appl Physiol (1985). 2000; 89:760–9.

47. Simon PM, Zurob AS, Wies WM, Leiter JC, Hubmayr RD. Entrainment of respiration in humans by periodic lung inflations: effect of state and CO(2). Am J Respir Crit Care Med. 1999; 160:950–60.
48. Meza S, Mendez M, Ostrowski M, Younes M. Susceptibility to periodic breathing with assisted ventilation during sleep in normal subjects. J Appl Physiol (1985). 1998; 85:1929–40.
49. Tobin MJ. Monitoring of pressure, flow, and volume during mechanical ventilation. Respir Care. 1992; 37:1081–96.
50. Jubran A, Van de Graaff WB, Tobin MJ. Variability of patient-ventilator interaction with pressure support ventilation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995; 152:129–36.

51. Terzi N, Pelieu I, Guittet L, Ramakers M, Seguin A, Daubin C, et al. Neurally adjusted ventilatory assist in patients recovering spontaneous breathing after acute respiratory distress syndrome: physiological evaluation. Crit Care Med. 2010; 38:1830–7.

52. Spahija J, de Marchie M, Albert M, Bellemare P, Delisle S, Beck J, et al. Patient-ventilator interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med. 2010; 38:518–26.

53. Xirouchaki N, Kondili E, Vaporidi K, Xirouchakis G, Klimathianaki M, Gavriilidis G, et al. Proportional assist ventilation with load-adjustable gain factors in critically ill patients: comparison with pressure support. Intensive Care Med. 2008; 34:2026–34.

54. Piquilloud L, Vignaux L, Bialais E, Roeseler J, Sottiaux T, Laterre PF, et al. Neurally adjusted ventilatory assist improves patient-ventilator interaction. Intensive Care Med. 2011; 37:263–71.

55. Kondili E, Prinianakis G, Alexopoulou C, Vakouti E, Klimathianaki M, Georgopoulos D. Respiratory load compensation during mechanical ventilation: proportional assist ventilation with load-adjustable gain factors versus pressure support. Intensive Care Med. 2006; 32:692–9.
Figure 1.
Schematic illustration of the respiratory system and the applied pressures. The respiratory system is presented as a balloon at passive functional residual capacity (FRC) (continuous line). Two dashed lines indicate volumes above (ΔV1) and below (ΔV2) FRC. In mechanically ventilated patients during inspiration ventilator pressure (Paw) and pressure developed by inspiratory muscles (PmusI) generate flow and the volume increases above passive FRC. The sum of these two pressures is dissipated to overcome elastic pressure (Pel), and resistive pressure (Pres). All these pressures have positive values in the equation of motion. Pressure developed by contraction of expiratory muscles (PmusE), elastic recoil pressure due to volume below passive FRC and resistive pressure due to V’E have negative values in the equation. V’I : inspiratory flow; Rrs: resistance of the respiratory system; V’E: expiratory flow; Ers: elastance of the respiratory system. Modified from Kondili et al. Br J Anaesth 2003;91:106-19, with permission of Oxford University Press [43].
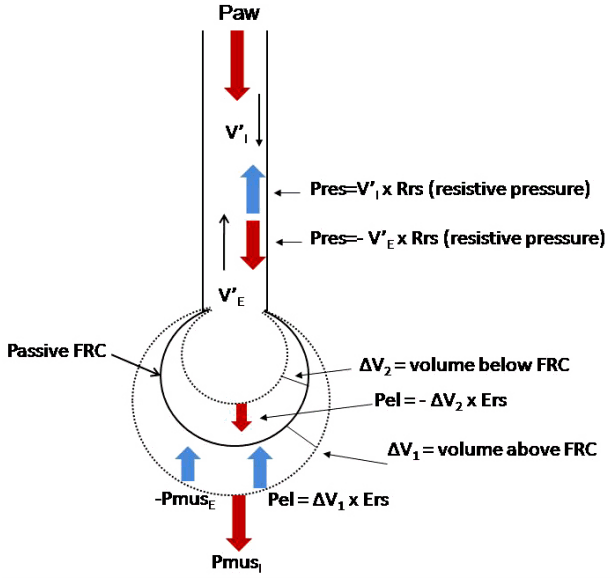
Figure 2.
Airway pressure (Paw), flow and esophageal pressure (Pes) time curves in a patient ventilated with pressure support ventilation. Observe that the second decrease in Pes, which represents inspiratory effort of the patient, is not followed by a mechanical breath. This is ineffective effort (IE) during expiration and is manifested by a slight decrease in Paw associated with a simultaneous decrease in expiratory flow (red arrows). Notice that the signal of flow distortion is much clearer than the corresponding Paw change. In every mechanical breath, there is a time lag between the start of neural inspiration (first dotted line) and the start of mechanical inspiration (second dotted line). This time lag is the triggering delay. Observe the spike early in expiratory flow (black arrows) after each breath that suggests high airway resistance and long-time constant causing incomplete exhalation (flow is not zero before the next breath). Dynamic hyperinflation causes triggering delay and, combined with a relatively weaker patient effort (second Pes deflection smaller than the others) leads to ineffective triggering.
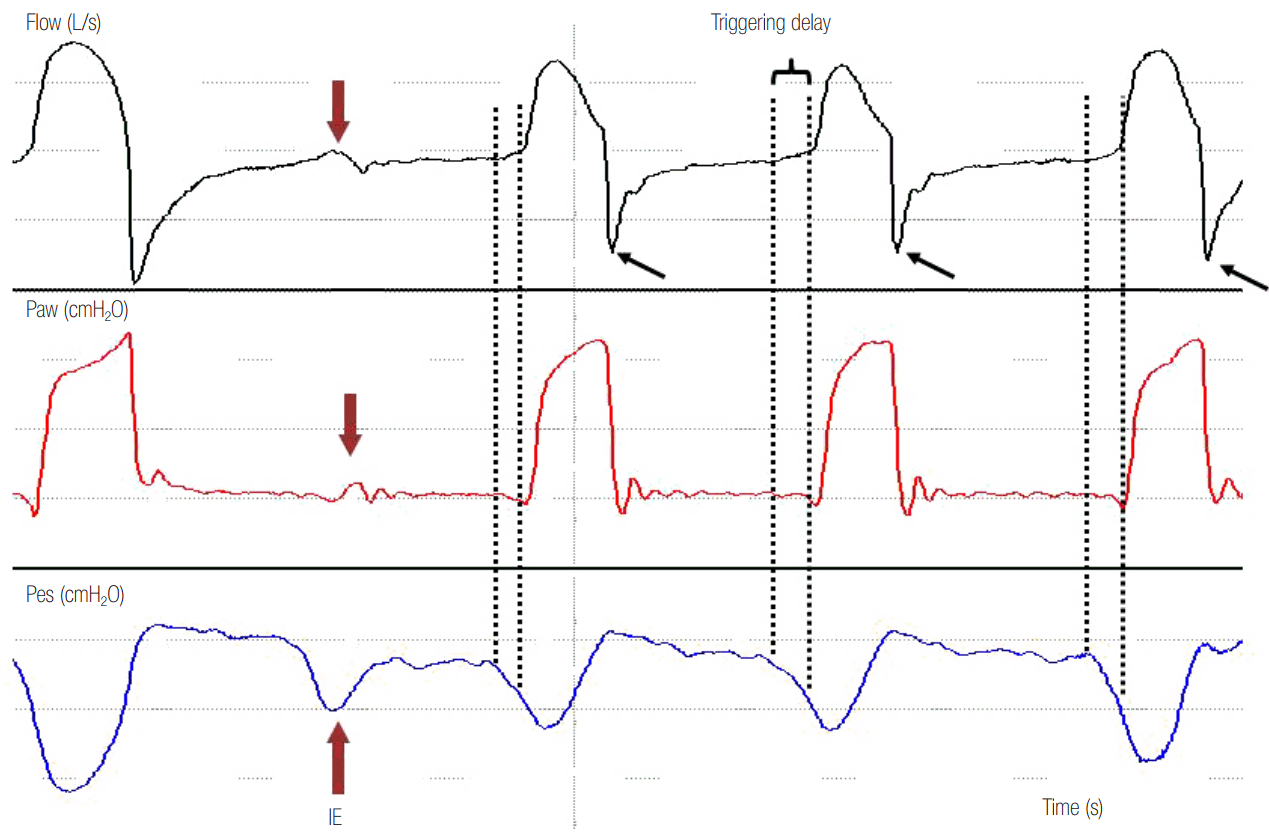
Figure 3.
Flow and esophageal pressure (Pes) time curves in a patient ventilated with pressure support ventilation. The start of neural inspiration (dotted line) is indicated by a rapid decrease in Pes associated with a rapid decrease in expiratory flow (expiratory flow returns rapidly to zero line). The two subsequent patient efforts are not accompanied by a mechanical breath and represent ineffective efforts (IE, red arrows). Both can be identified by the associated flow distortion. The first IE takes place during mechanical inspiration and causes an increase in inspiratory flow waveform. The second IE happens during expiration and is manifested by a decrease in expiratory flow. The spike early in expiratory flow (black arrow) due to high airway resistance and the incomplete exhalation (flow is not zero before the next breath) are signs of dynamic hyperinflation.
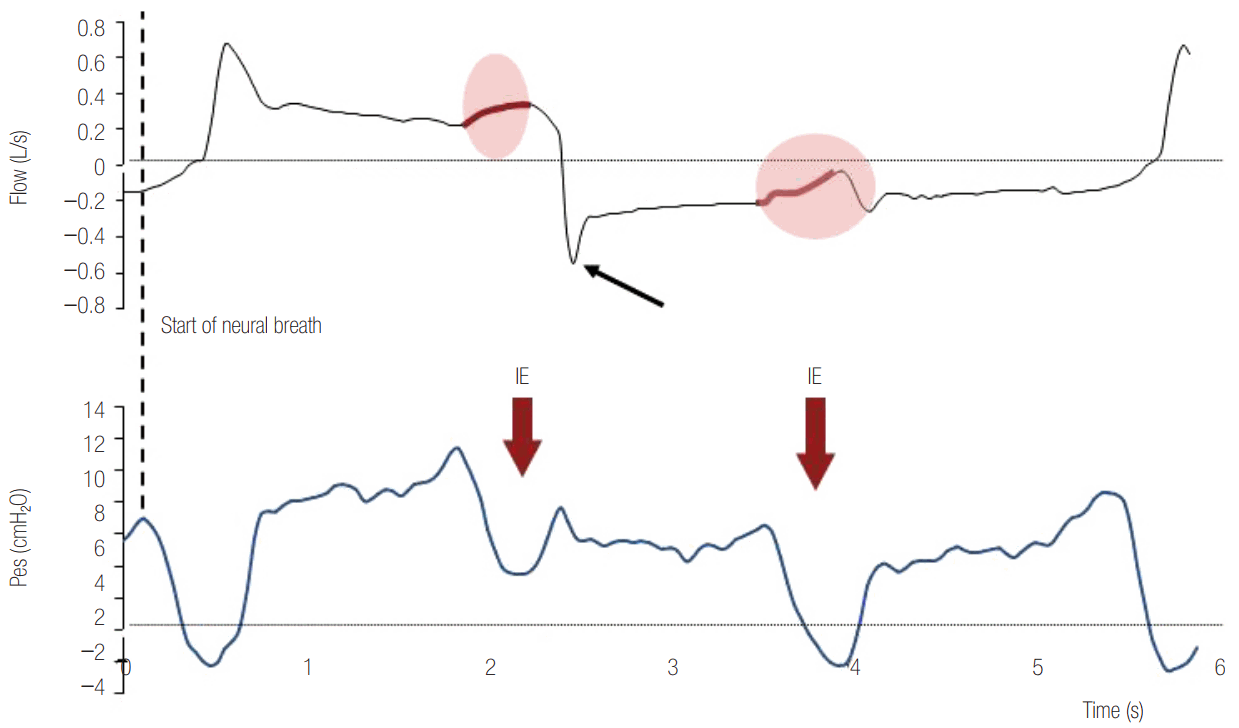
Figure 4.
Airway pressure (Paw), flow and transdiaphragmatic pressure (Pdi) time curves of a patient ventilated on pressure support ventilation are illustrated. As indicated by the absence of Pdi increase, there is no inspiratory effort before the second mechanical breath (autotriggered breath, see blue shaded area). We can observe that, in comparison to patient-triggered breaths, where a decrease in Paw is observed before the start of mechanical inflation (grey shaded areas), there is no distortion in the Paw- (no decrease in Paw) and flow-time curve in the autotriggered breath. Moreover, the shape of the inspiratory flow-time curve is different compared to that of patient-triggered breaths. Notice the absence of dynamic hyperinflation in this patient (expiratory flow returns to zero after each breath).
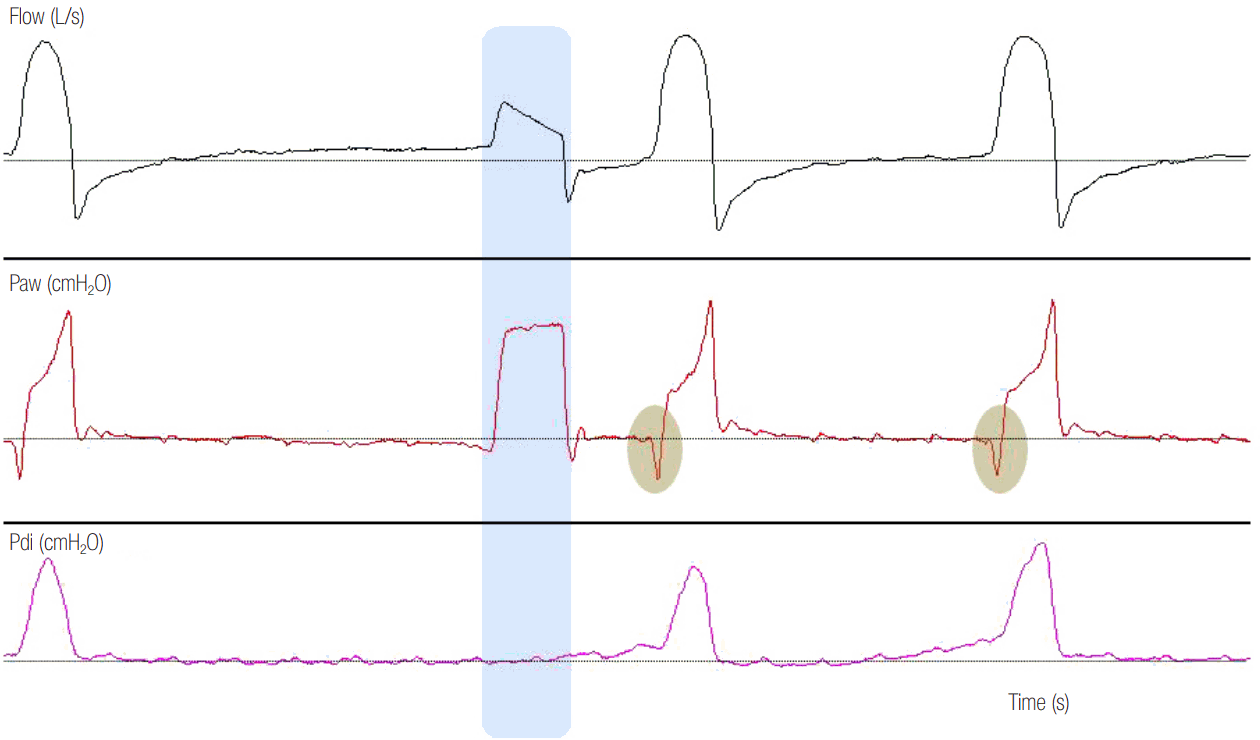
Figure 5.
Delayed opening of the expiratory valve. Flow, airway pressure (Paw), gastric pressure (Pgas) and esophageal pressure (Pes) time waveforms in a patient ventilated with pressure support ventilation. There is a significant time delay (blue shaded area) between the end of neural inspiration, recognized by a rapid increase in Pes, and the end of mechanical inspiration, signified by the termination of inspiratory flow (inspiratory flow equals zero). Observe the rapid increase of Paw towards the end of mechanical inspiration, indicating inspiratory muscle relaxation.
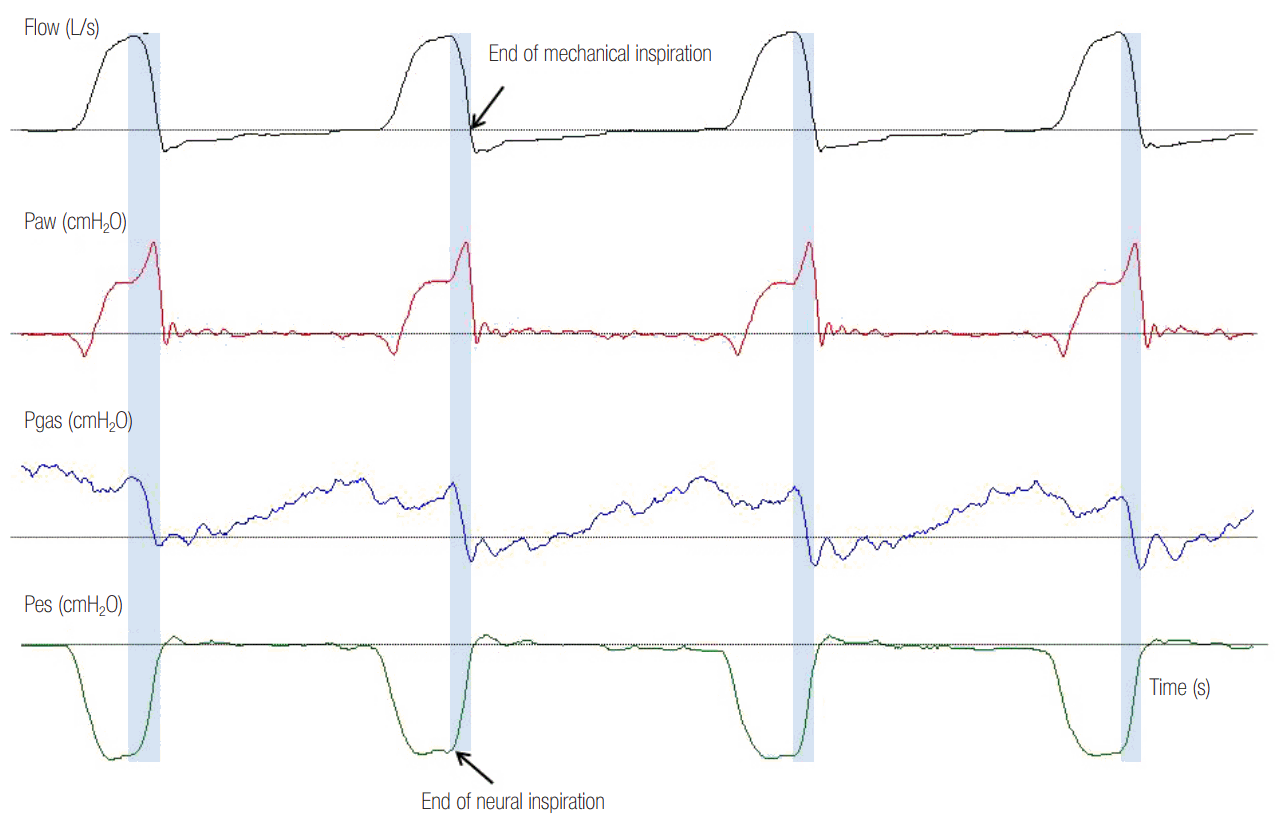
Figure 6.
Reverse triggering in a patient ventilated with assist pressure control ventilation. There is an inspiratory effort of the patient (dotted lines), as evidenced by the rapid increase in electromyographic activity of the diaphragm (EAdi) after every mechanical inflation (1:1 relationship). The time interval between the initiation of mechanical and neural inspiration is fixed. Indirect evidence of patient inspiratory activity during mechanical inflation is the notch in Paw (grey shaded area). Paw: airway pressure; VT: tidal volume.
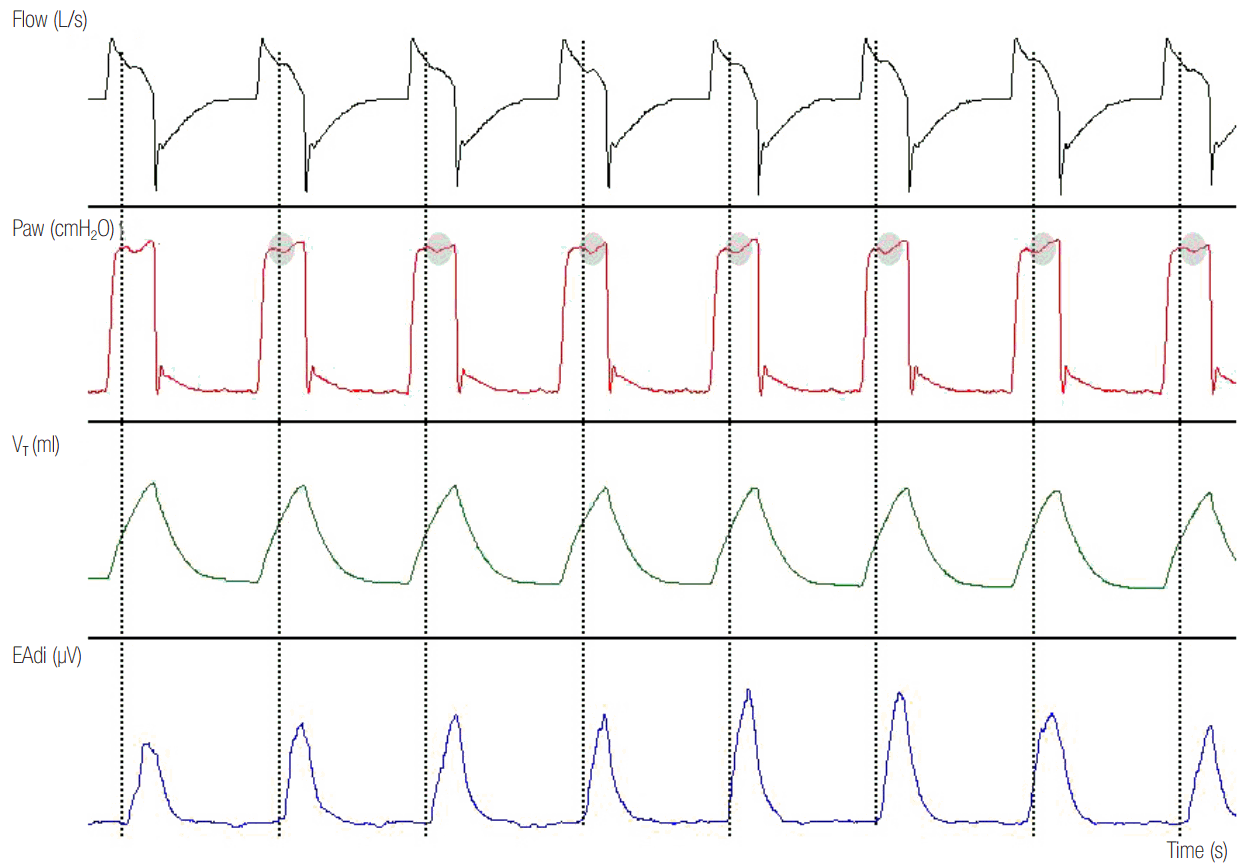
Figure 7.
Reverse triggering in a patient ventilated with assist volume control ventilation. Esophageal pressure (Pes) decrease reveals patient inspiratory efforts (blue line) after every mechanical inflation in 1:1 relationship. Indirect evidence of patient inspiratory activity during mechanical inflation is the flow distortion (grey shaded area) and the disappearance (blue arrows) of plateau airway pressure (Paw) in the flow-time and Paw-time waveform, respectively. In this patient, a reverse triggered breath was strong enough to trigger the ventilator at the end of the mechanical inspiration, causing breath stacking (red shaded area). Inflated tidal volume (VT) during breath stacking increased from 444 ml to 800 ml (double arrow).
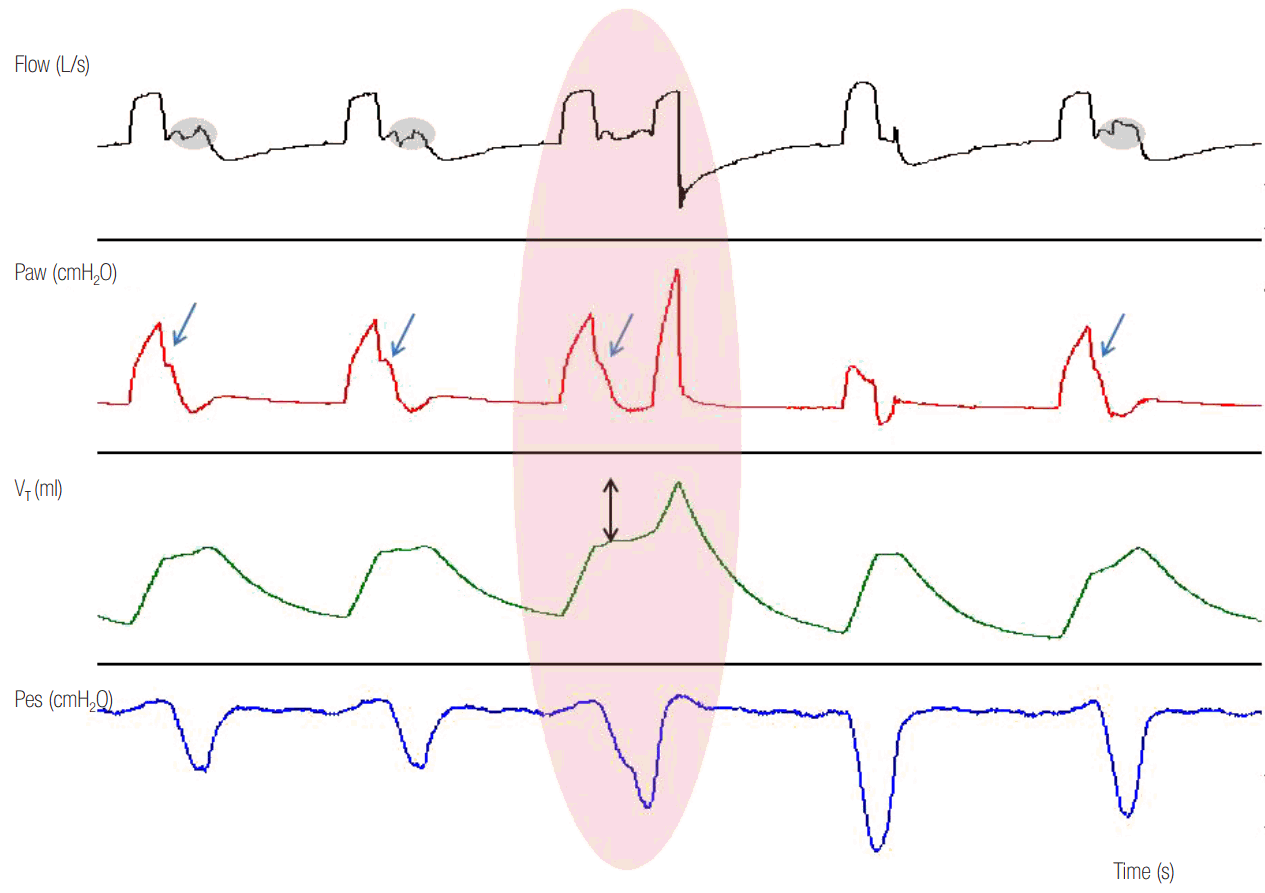
Figure 8.
Flow, airway pressure (Paw), esophageal pressure (Pes) and transdiaphragmatic pressure (Pdi) time waveforms in a patient ventilated with pressure support ventilation. Observe the vigorous contraction of inspiratory muscles (Pdi increase) during the mechanical inspiration. The magnitude of this contraction causes a rounded inspiratory flow and a large decrease of Paw (gray shaded area) from the expected square-shaped form during inspiration. Rounded flow and Paw decrease are signs of low ventilator assist with respect to patients ventilator demands.
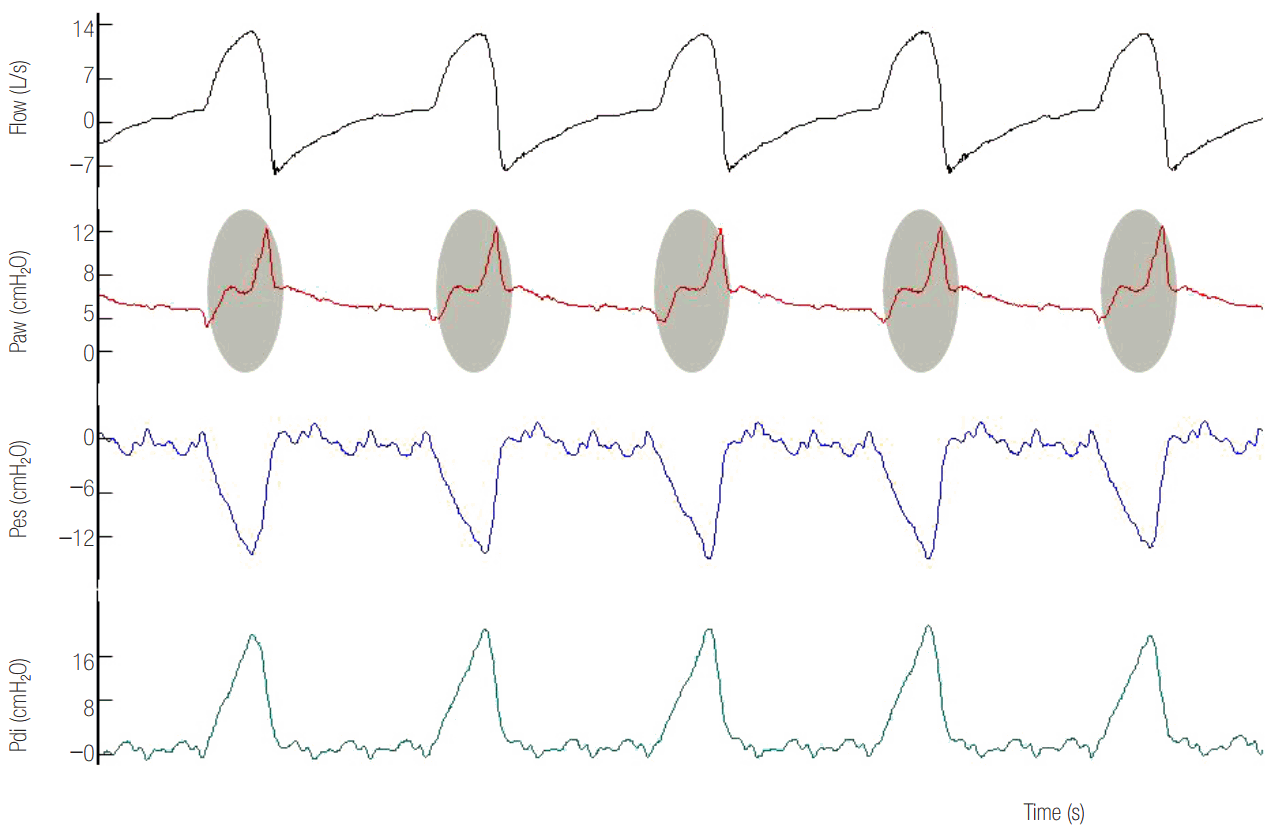
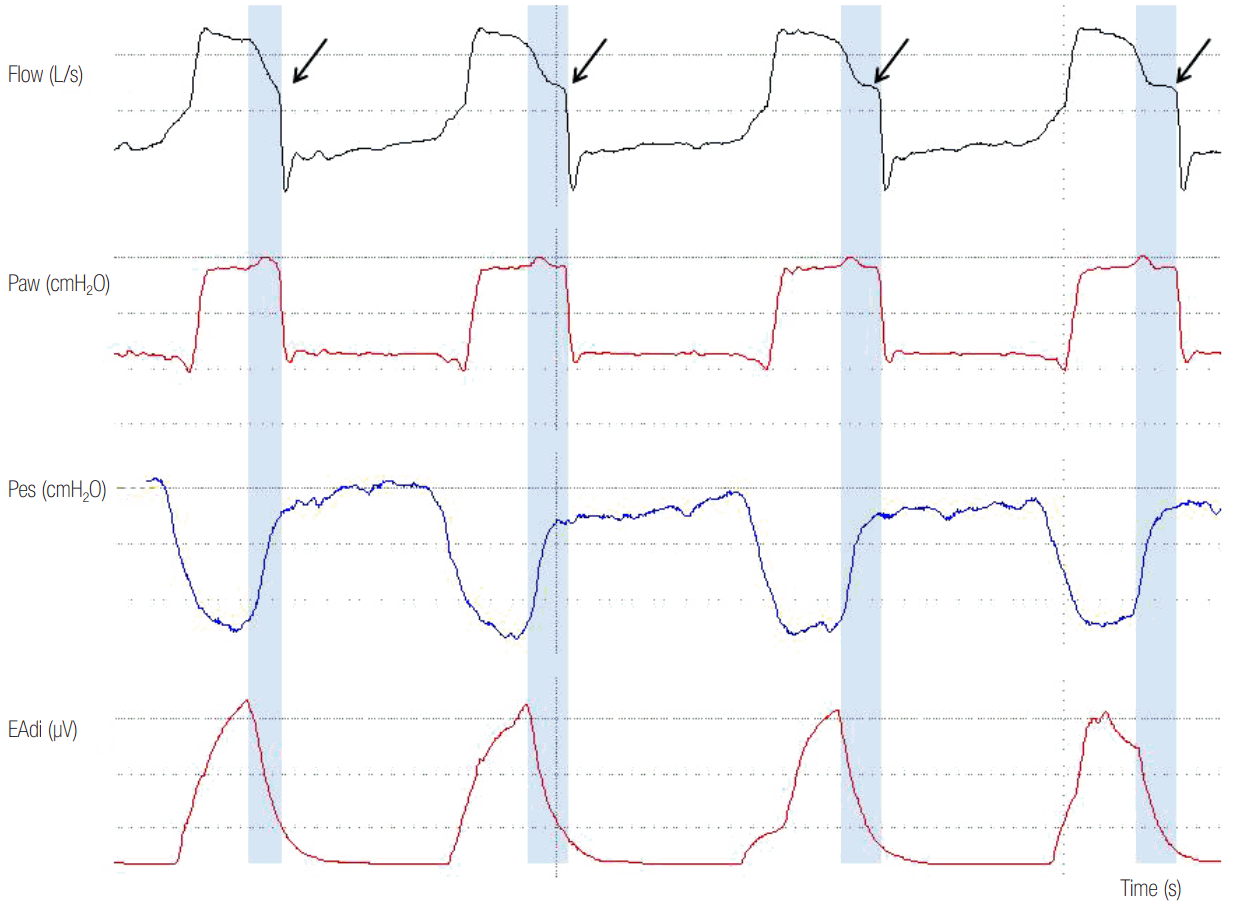
Figure 9.
High assist in a patient ventilated with pressure support ventilation. Observe the square shaped airway pressure (Paw) and the abrupt decrease in inspiratory flow to flow threshold for cycling off towards the end of inspiration (arrows). There is also a significant cycling off delay (blue shaded area), seen often at high assist levels. Esophageal pressure (Pes) and electromyographic activity of the diaphragm (EAdi) decrease rapidly but mechanical inflation continues. Importantly, expiratory muscles contract during the whole expiration.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download