Spontaneous echo contrast (SEC) is often observed in patients with mitral stenosis, atrial fibrillation, cardiomyopathy, or a ventricular aneurysm [1]. SEC is a smoke-like echo density observed on echocardiograms, and is caused by increased red blood cell aggregation during low-flow states. It is also a risk factor of thromboembolism [2]. SEC can be observed in patients with severe ventricular dysfunction receiving venoarterial extracorporeal membrane oxygenation (VA-ECMO). We present a case in which left ventricular-SEC (LV-SEC) was mistaken for a LV thrombus during VA-ECMO for severe LV dysfunction.
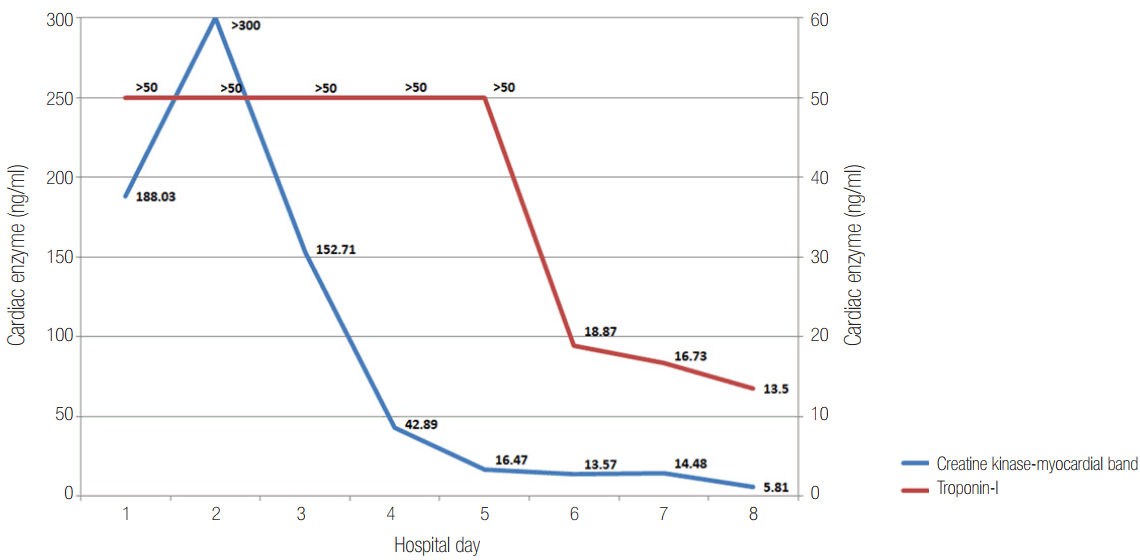
A 36-year-old female patient diagnosed with acute fulminant myocarditis was provided VA-ECMO support on hospital day (HD) 1. Briefly, VA-ECMO (Rota-Flow; Maquet Inc., Hirrlingen, Germany) was implanted in the right femoral artery (15-French arterial cannula) and the left femoral vein (20-French venous cannula). Her height and body weight are 163 cm and 52 kg (body surface area, 1.53 m2). VA-ECMO was initiated with a circuit flow of 3.5 L/min (cardiac index, 2.3 2L/min/m2). Her creatine kinase-myocardial band and troponin-I levels at admission were 188.03 ng/ml (normal range, 0 to 5 ng/ml) and >50.0 ng/ml (normal range, 0 to 0.78 ng/ml), respectively. Impaired ventricular function (ejection fraction, 22%) suspected as acute fulminant myocarditis was detected by transthoracic echocardiography (TTE) at admission.
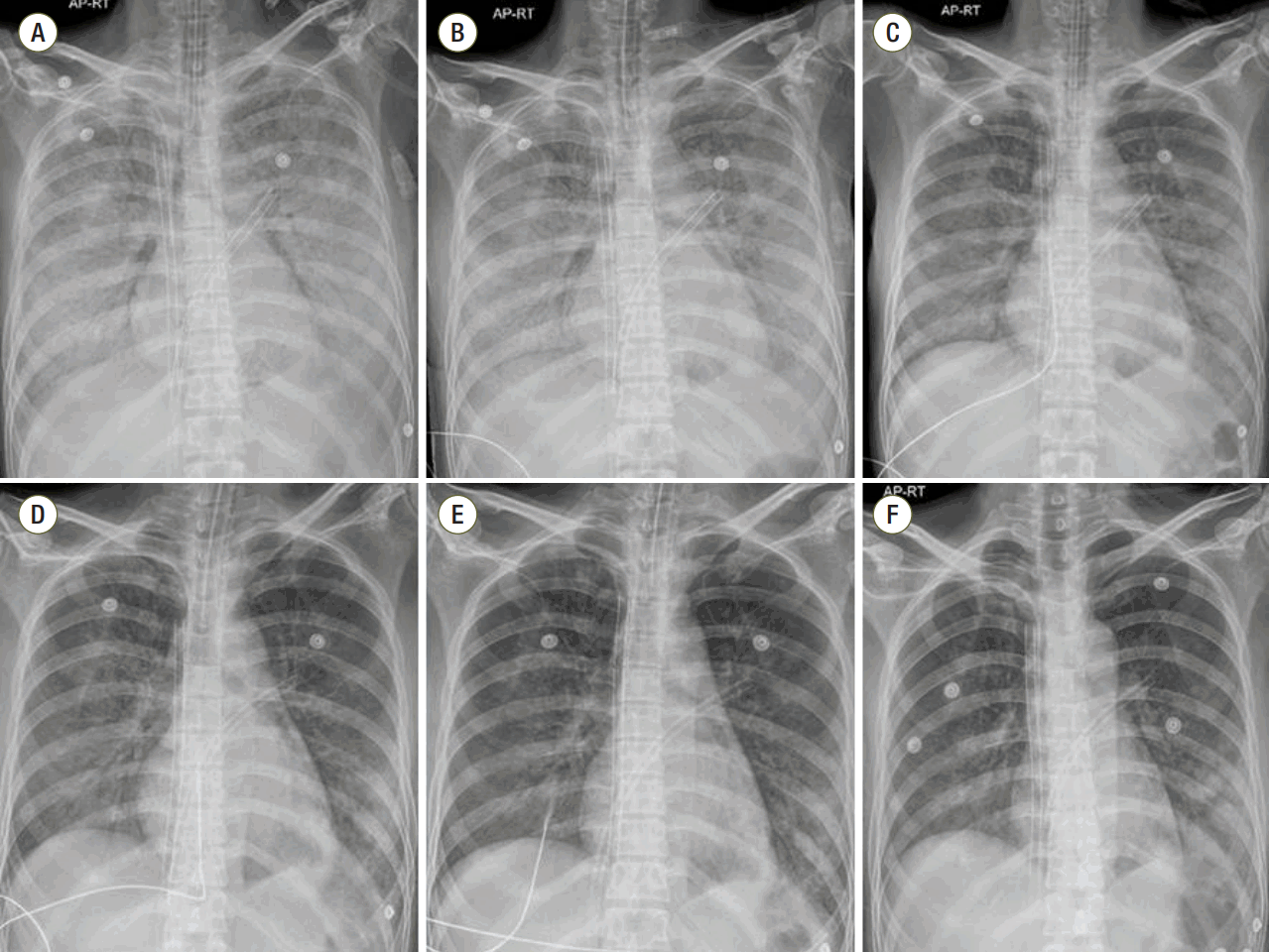
TTE revealed decreased LV function (ejection fraction, 10%) with mild mitral regurgitation (grade II) immediately after VA-ECMO. Opening of the aortic valve and arterial pulsatility were not observed. Pulmonary edema was aggravated on HD 4. Left atrial (LA) decompression was achieved using a LA catheter (20-French femoral venous cannula) by balloon atrial septostomy through the right femoral vein. Pulmonary edema and cardiomegaly improved after LA decompression (Figure 1) and cardiac enzymes levels were reduced (Figure 2).
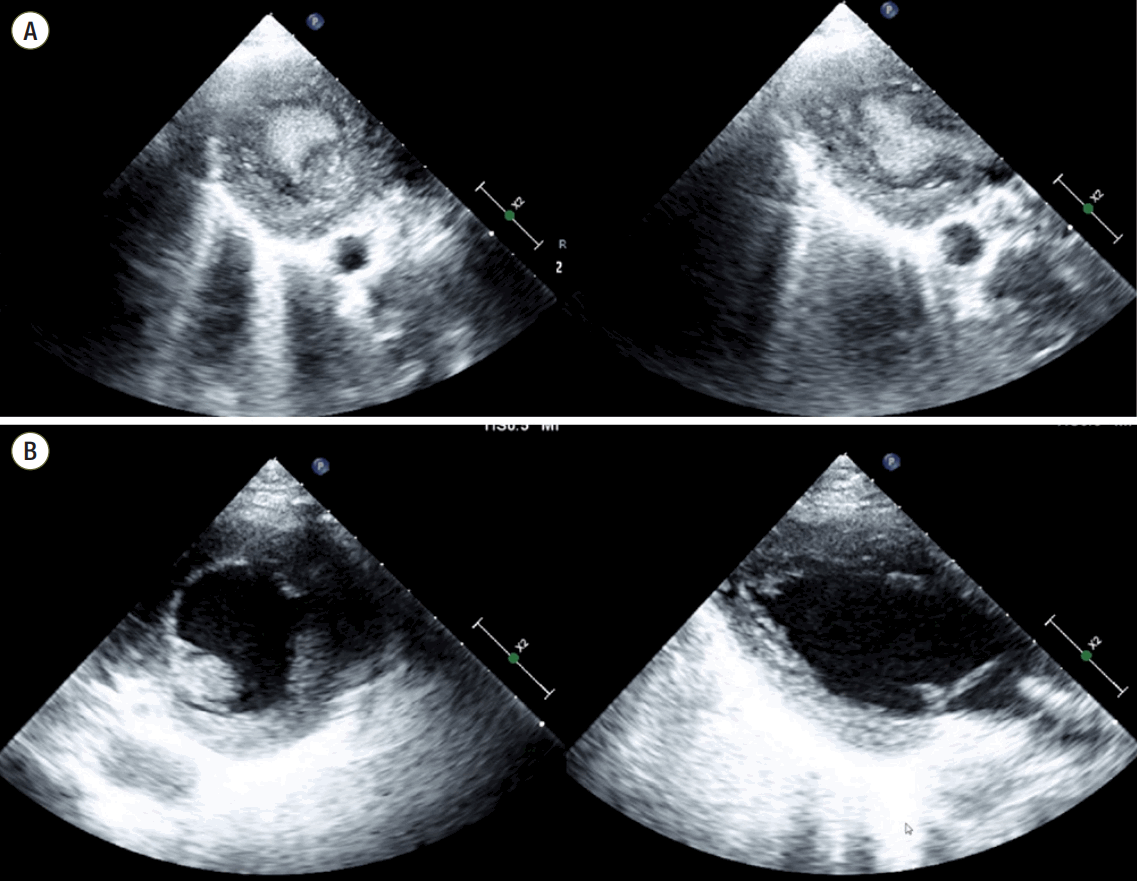
Input and output were controlled to improve pulmonary edema; about 5,000 ml volume was removed from HD 5 to 10. On HD 11, ECMO flow was abruptly reduced from 3.2 to 1.6 L/min, and hyperechogenic material was detected in the LV using a portable TTE (Figure 3A). In view of the abrupt ECMO flow reduction, we considered LV thrombus, but after infusing normal saline (500 ml) ECMO flow recovered and the hyperechogenic material disappeared (Figure 3B). We realized that LV-SEC was misdiagnosed as LV thrombus. At that time, her activated prothrombin time was 100 seconds, and fortunately there was no evidence of thromboembolic complications. Her cardiac function then recovered and on HD 14 VA-ECMO was weaned without embolic or bleeding complications.
VA-ECMO administered using peripheral cannulation induces retrograde blood flow and increases afterload. If LV dysfunction is severe, the aortic valve may not open against a high afterload and pulsatility may disappear. In such cases, blood stasis and SEC are observed [3]. In the absence of pulsatility, TTE is important to ensure the presence of swirling. Excessive volume removal and LA venting can aggravate SEC because preload is lowered and the LV cavity is emptied [4]. Furthermore, if ECMO flow decreases abruptly, SEC may be mistaken for thrombus. In this context, cautious interpretation of echocardiographic finding based on the clinical situation is important to avoid invasive procedures, such as exploratory cardiac surgery.
It is also important to reduce the afterload for improving the pulsatility. Combining ECMO with intra-aortic balloon pump may have benefit in term of decreasing the afterload [5]. Vasodilator medications, such as nitroprusside, nitroglycerin, calcium channel blockers can reduce the afterload as well. The flow of VA-ECMO should also be adjusted while confirming the perfusion status and mean arterial pressure [6].
Myocarditis is a hypercoagulable state induced by a systemic inflammatory process [7], and thus adequate anticoagulation therapy is required during VA-ECMO to prevent thrombotic complications. In particular, when SEC is observed in a patient with severe LV dysfunction, the risk of these complications increases. In the described case, we performed VA-ECMO for acute fulminant myocarditis with severe LV dysfunction, and followed this with daily echocardiography to identify swirling or SEC. Target activated prothrombin time of heparin therapy was maintained at a high level (>70 seconds) because the aortic valve did not open and swirling was observed. Furthermore, if our patient had not been given inappropriate anticoagulation therapy when SEC was present, complications of thrombus would certainly have occurred.
In conclusion, VA-ECMO in patients with acute fulminant myocarditis and severe LV compromise provides meaningful life support and facilitates the recovery of cardiac function. However, SEC can occur in the absence of pulsatility or aortic valve opening, and severe volume restriction and LA venting might increase the risk of LV-SEC by emptied the LV cavity. LV-SEC may be mistaken for thrombus when ECMO flow abruptly reduced, and thus, repeat evaluation using TTE and cautious interpretation of echocardiographic finding are required. On the other hand, in patients with SEC, proper anticoagulation therapy is important to prevent complications of thrombus.
Go to : 
References
1. Black IW. Spontaneous echo contrast: where there’s smoke there’s fire. Echocardiography. 2000; 17:373–82.

2. Black IW, Hopkins AP, Lee LC, Walsh WF. Left atrial spontaneous echo contrast: a clinical and echocardiographic analysis. J Am Coll Cardiol. 1991; 18:398–404.

3. Unai S, Nguyen ML, Tanaka D, Gorbachuk N, Marhefka GD, Hirose H, et al. Clinical significance of spontaneous echo contrast on extracorporeal membrane oxygenation. Ann Thorac Surg. 2017; 103:773–8.

4. Pepi M, Evangelista A, Nihoyannopoulos P, Flachskampf FA, Athanassopoulos G, Colonna P, et al. Recommendations for echocardiography use in the diagnosis and management of cardiac sources of embolism: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr. 2010; 11:461–76.

5. Ma P, Zhang Z, Song T, Yang Y, Meng G, Zhao J, et al. Combining ECMO with IABP for the treatment of critically ill adult heart failure patients. Heart Lung Circ. 2014; 23:363–8.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download