We report a case of 20-year-old female patient with ofloxacin-induced anaphylaxis mediated by IgG4 antibody.
One of second-generation fluoroquinolone, ofloxacin is prescribed widely to treat bacterial infections. Reports of serious hypersensitivity reactions to quinolone are increasing due to high consumption worldwide [1].
The pathogenesis of anaphylaxis caused by ofloxacin is not yet fully understood, as it is not commonly reported. There has been a report demonstrating high serum specific IgE levels to ofloxacin-human serum albumin (HSA) conjugate using enzymelinked immunosorbent assay (ELISA), enzyme-linked immunospecific assay [2]; however, this is the first report to suggest an IgG4-mediated but not IgE-mediated mechanism in a patient with ofloxacin-induced anaphylaxis.
A 20-year-old female with allergic rhinitis was referred to our department for the evaluation of anaphylaxis because she had experienced generalized urticaria and dyspnea with wheezing, and hoarseness within 1 hour after oral ingestion of ofloxacin 100 mg. It was her first ingestion of ofloxacin. The patient had a history of acute, generalized urticaria and drug allergy to nonsteroidal anti-inflammatory drugs. Her mother had a history of bronchial asthma. At the initial visit, no abnormal findings were noted on physical examination and radiography/spirometry results. Her serum total IgE level was 42 kU/L. Allergy skin prick tests showed positive responses to Dermatophagoides farinae and D. pteronyssinus, but negative responses to ofloxacin at concentrations of 0.1−10 mg/ml, with a positive control being a mean wheal size of 4 mm to histamine.
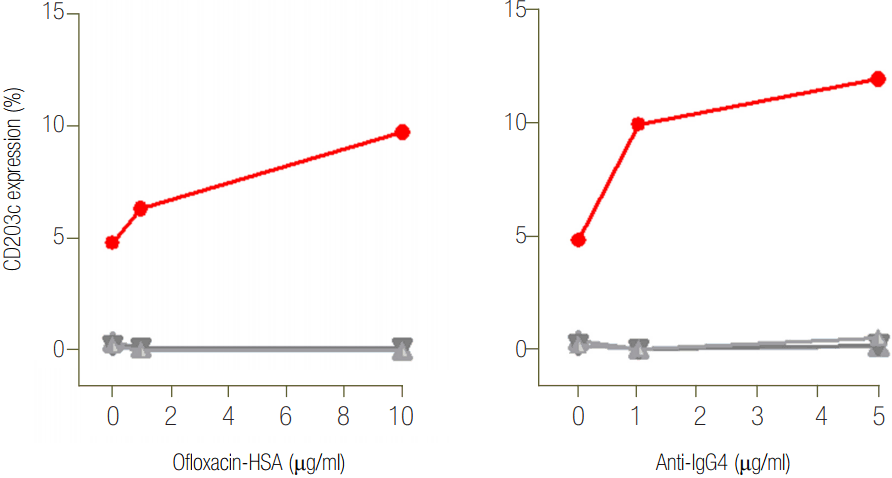
Ofloxacin-HSA conjugate was conducted to investigate immunologic mechanisms to detect serum specific IgE antibody to ofloxacin-HSA conjugate using ELISA as previously described [2]. When the positive cutoff value was determined from the mean +3 standard deviation absorbance values of 20 nonatopic healthy controls that never experienced any other drug allergy according to medical records, serum specific IgE antibody was not detected in the patient’s serum, while high serum specific IgG4 antibody was detected as shown in Figure 1. A basophil activation test (BAT) was done with addition of ofloxacin and anti-IgG4 antibody using peripheral basophils from the patient and three healthy controls to confirm specific IgG4-mediated mechanisms. A significant upregulation of CD203c, a marker of activated basophils, was noted with serial additions of ofloxacin-HSA (from 6.35% to 9.7%), while no changes were noted in three healthy controls (Figure 2).
Until now, anaphylaxis cases caused by ciprofloxacin, one of the most frequently prescribed oral fluoroquinolones in the U.S. and worldwide, have occasionally been reported [3,4]. The frequency of ofloxacin prescription as an alternative therapy is expected to increase due to a high incidence of drug-resistant tuberculosis in Asia.
Until now, immediate reactions such as urticaria and anaphylaxis were reported as a phenotype of hypersensitivity reactions to quinolone, although frequencies have been reported to be less than 2% [1]. An IgE-mediated reaction is known as a major pathogenetic mechanism of immediate hypersensitivity to quinolone. Using sepharoseradioimmunoassay for the determination of specific IgE in the serum, a previous study reported an IgE-mediated mechanism in 12 of 38 (31.5%) patients with anaphylaxis and urticaria as well as serum specific IgE to ciprofloxacin, moxifloxacin, and levofloxacin [5]. Another study showed in 30 of 55 (54.5%) patients with immediate reactions to eight quinolones including ofloxacin detected by serum specific IgE [6]. In cases of ofloxacin-induced anaphylaxis, our previous study reported four of five (80%) patients with serum specific IgE to ofloxacin-HSA conjugate using ELISA [2].
Previous studies have suggested involvement of non-IgE mediated mechanisms in patients with quinolone-induced immediate hypersensitivity reactions [1,7]. Nam et al. [8] reported one patient with cefotetan-induced anaphylaxis that was mediated by IgG antibody. The non-IgE mediated mechanisms of anaphylaxis are not well understood in cases of quinolone-induced anaphylaxis. Since IgG-mediated immediate anaphylaxis through the low-affinity IgG receptor Fcγ-receptor was introduced in a murine model [9], IgG-mediated alternative pathways are suggested, as basophil to release platelet-activating factor upon stimulation with allergen-IgG complexes [10]. It is possible that alternative pathways could be mediated by basophils, IgG, IgG receptor, and platelet-activating factor, particularly a high level of serum IgG, but not specific IgE to relevant allergens. The BAT is a useful diagnostic method for immediate-type drug allergy based upon different activation markers, being mostly CD203c and CD63 involving IgE-dependent and IgE-independent basophil-mediated reactions [11-13]. The sensitivity of the BAT to quinolone has recently been reported to 71.1% [5]. In the present study, we presumed that specific IgG4 antibody may play a role in the activation of basophil as a significant mediator through an alternative mechanism in that there were a high serum specific IgG4 and no serum specific IgE to ofloxacin-HSA conjugate and a significant upregulation of basophils in response to ofloxacin and anti-IgG4 antibody.
Anaphylaxis is a serious reaction that may lead to death and prompt management is crucial [14]. The mainstay of anaphylaxis treatment is to restore and maintain vital signs by early administration of intramuscular epinephrine along with antihistamine and corticosteroid. Regardless of allergen or mechanism of anaphylaxis, mast cell and basophil are activated to commence and amplify allergic reactions, and release various inflammatory mediators such as tryptase and histamine to cause anaphylaxis reactions [15].
In conclusion, this is a case of ofloxacin-induced anaphylaxis through an IgG4-mediated but not IgE-medicated basophil activation mechanism.
References
1. Blanca-López N, Andreu I, Torres Jaén MJ. Hypersensitivity reactions to quinolones. Curr Opin Allergy Clin Immunol. 2011; 11:285–91.

2. Nam YH, Kim JE, Kim SH, Jin HJ, Hwang EK, Shin YS, et al. Immunologic evaluation of ofloxacin hypersensitivity. Allergy Asthma Immunol Res. 2012; 4:367–9.

3. Hicks LA, Bartoces MG, Roberts RM, Suda KJ, Hunkler RJ, Taylor TH Jr, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015; 60:1308–16.

4. Jones SC, Budnitz DS, Sorbello A, Mehta H. USbased emergency department visits for fluoroquinolone-associated hypersensitivity reactions. Pharmacoepidemiol Drug Saf. 2013; 22:1099–106.

5. Aranda A, Mayorga C, Ariza A, Doña I, Rosado A, Blanca-Lopez N, et al. In vitro evaluation of IgE-mediated hypersensitivity reactions to quinolones. Allergy. 2011; 66:247–54.

6. Manfredi M, Severino M, Testi S, Macchia D, Ermini G, Pichler WJ, et al. Detection of specific IgE to quinolones. J Allergy Clin Immunol. 2004; 113:155–60.

7. Lobera T, Audícana MT, Alarcón E, Longo N, Navarro B, Muñoz D. Allergy to quinolones: low crossreactivity to levofloxacin. J Investig Allergol Clin Immunol. 2010; 20:607–11.
8. Nam YH, Hwang EK, Ban GY, Jin HJ, Yoo HS, Shin YS, et al. Immunologic evaluation of patients with cefotetan-induced anaphylaxis. Allergy Asthma Immunol Res. 2015; 7:301–3.

10. Tsujimura Y, Obata K, Mukai K, Shindou H, Yoshida M, Nishikado H, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008; 28:581–9.

11. Gómez E, Torres MJ, Mayorga C, Blanca M. Immunologic evaluation of drug allergy. Allergy Asthma Immunol Res. 2012; 4:251–63.

12. Kim SY, Kim JH, Jang YS, Choi JH, Park S, Hwang YI, et al. The basophil activation test is safe and useful for confirming drug-induced anaphylaxis. Allergy Asthma Immunol Res. 2016; 8:541–4.

13. Song JW, Jang YS, Jung MC, Kim JH, Choi JH, Park S, et al. Levodropropizine-induced anaphylaxis: case series and literature review. Allergy Asthma Immunol Res. 2017; 9:278–80.





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download