Abstract
Diffuse alveolar hemorrhage (DAH) is associated with severe outcomes. We report a case of acute respiratory failure that required mechanical ventilation and was clinically and pathologically diagnosed as DAH related to exposure to organic dust. A 39-year-old man, who had visited a warehouse to grade beans for purchase, was referred to our hospital for impending respiratory failure. His initial radiographic examinations revealed diffuse bilateral ground-glass opacities in his lungs and bronchoalveolar lavage resulted in progressively bloodier returns, which is characteristic of DAH. He underwent bedside open lung biopsy of his right lower lobe in the intensive care unit. Biopsy results revealed DAH and organization with accumulation of hemosiderin-laden macrophages and a few fibroblastic foci. The patient was treated with empirical antibiotics and high-dose corticosteroids and successfully weaned from mechanical ventilation. DAH might be considered in the differential diagnosis of patients with acute respiratory failure after exposure to organic particles.
Diffuse alveolar hemorrhage (DAH) is a syndrome characterized by the accumulation of intra-alveolar red blood cells originating from the alveolar capillaries.[1] The clinical presentation of this syndrome includes hemoptysis, anemia, and diffuse bilateral infiltration on chest radiography. Severe cases can present as respiratory failure. The etiology of DAH is variable and includes small vessel vasculitis, connective tissue disease, and diffuse alveolar damage caused by certain drugs and infections. [1] We present the case report of a patient with histopathologically confirmed DAH related to organic dust exposure, who presented with acute respiratory failure requiring mechanical ventilation.
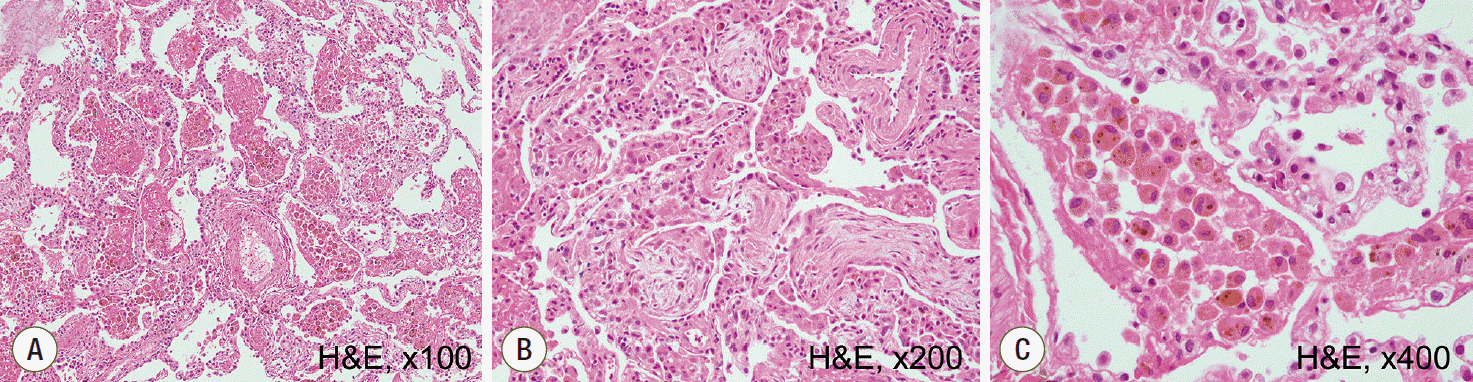
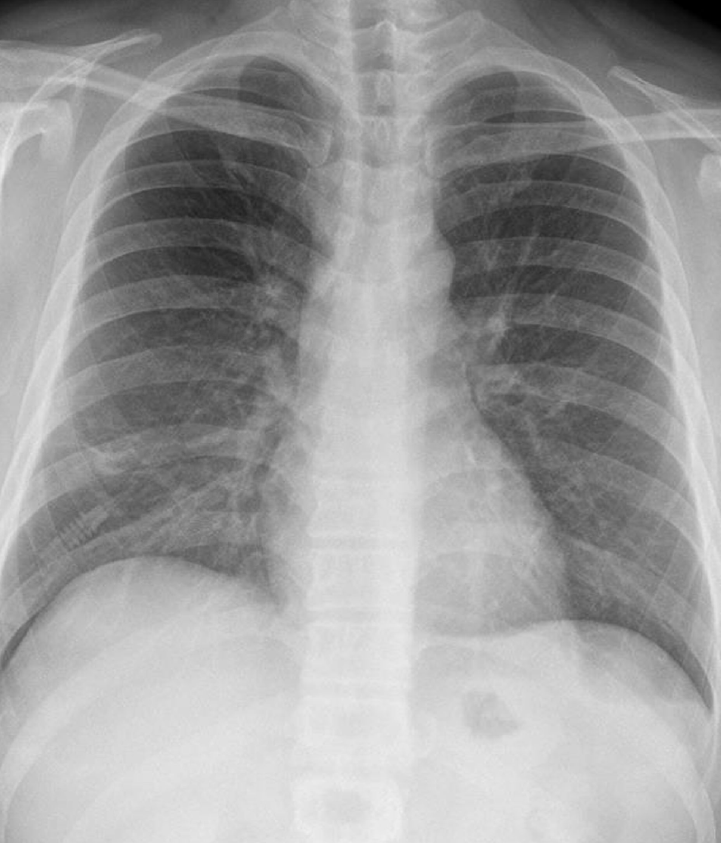
A 39-year-old man working in the Korea National Agricultural Cooperative Federation was referred to our hospital for impending respiratory failure with hemoptysis. He had been working as an office worker in the company for 10 years. He was a current smoker with a 15 pack-year smoking history and did not have any history of other disease and medication. Two months ago, his job required him to grade beans for purchase and thus visit warehouses to assess the quality of the beans. Approximately 10 days after he started visiting the warehouses, he began to cough with expectoration of small amount of blood-tinged sputum (BTS) and reported a tactile fever. The symptoms did not improved after coming home from work. As days go by, the BTS became more severe, and finally he developed dyspnea. Four days before admission to our hospital, he visited the emergency department of a regional hospital for dyspnea and hemoptysis of about 100 ml. He denied any other systemic symptoms such as arthralgia, myalgia, and skin rash. He also denied travel history. His chest radiography showed bilateral infiltration. Computed tomography (CT) showed multiple ground glass opacities and consolidations with a centrilobular pattern involving both lungs (Fig. 1), which suggested diffuse alveolar hemorrhage or acute hypersensitivity pneumonitis (HP). At the time of admission, his blood pressure was 172/82 mmHg and body temperature was 37℃. His initial laboratory findings were as follows: a partial pressure of arterial oxygen (PaO2) of 60.3 mmHg, partial pressure of arterial carbon dioxide (PaCO2) of 35.4 mmHg, a white blood cell (WBC) count of 15,130/μL, a hemoglobin level of 9.4 g/dL, a platelet count of 272,000/μL, and a C-reactive protein level of 0.84 mg/dL. A procalcitonin level was 0.05 ng/mL. The prothrombin time, activated partial thromboplastin time, and fibrinogen level were within the normal range. Urinary tests for pneumococcal and Legionella antigen were negative. Serological test for Mycoplasma pneumoniae, Leptospira, and Hantan virus were was also all negative. Real-time polymerase chain reaction assays for detection of respiratory viruses (parainfluenza virus, respiratory syncytial virus, and coronavirus) were all negative. The serologic tests for rheumatoid factor, fluorescent antinuclear antibody, anti-neutrophil cytoplasmic antibody, and cryoglobulin were all negative. He was treated with empirical antibiotics (piperacillin/tazobactam and levofloxacin) since admission and was given methylprednisolone at a dose of 40 mg/day for the first 2 days and 60 mg on the third day. However, the diffuse bilateral infiltrations on his chest radiograph progressed rapidly, with PaO2 levels that decreased over 3 days. He was referred to our hospital while on high flow nasal cannula with a fraction of inspired oxygen (FiO2) of 0.7. We continued the empirical antibiotics and methylprednisolone (60 mg/day). He was intubated on his second hospital day. We performed portable bronchoscopy in the intensive care unit (ICU), which revealed blood clots in the trachea and both bronchi. The fluid returned from subsequent bronchoalveolar lavages (BALs) become progressively bloodier, which is a typical finding of DAH (Fig. 2). BAL recovered 416,000 red blood cells/μL and 2,620 WBCs/μL with a differential of 90% neutrophils, 4% lymphocytes, 4% monohistiocytes, and 2% eosinophils. An elevated CD4/CD8 ratio of 39/16 in BAL fluid was observed. After bronchoscopy, his oxygen saturation dropped below 86% while on a mechanical ventilator with the following settings: FiO2 of 1.0, a positive end-expiratory pressure of 10 cmH2O, and 10 ppm of nitrous oxide. Therefore, we placed him in prone position for 16 hours per day for 2 days. The result of quantitative bacterial culture from BAL fluid revealed no growth of any microorganism, and the results of fungal and mycobacterial culture were negative. The results of polymerase chain reaction for respiratory virus panel in BLA fluid were also all negative. We started steroid pulse therapy (750 mg of methylprednisolone) on hospital day 3 and continued it through hospital day 5. On hospital day 4, he underwent bedside open lung biopsy in the ICU to evaluate the etiology of DAH, because we found all serologic tests negative and no evidence of systemic disease.[1] Before starting operation, we set FiO2 of 1.0 to maintain oxygen saturation as 100% for at least 5 min. We turned the ventilator off during the procedure until the oxygen saturation decreased to 92-93% (about 3 min), then we turned the ventilator on again to improve oxygen saturation. We repeated the operation of the ventilator during the surgery. The thoracic surgeon made the incision at the 5th intercostal space on anterior axillary line for the working window. After the lungs collapsed, the surgeon performed wedge resection of the right lower lobe without any significant complications. Starting on hospital day 5, an improvement in the PaO2/FiO2 ratio was observed. He was successfully weaned from mechanical ventilation on hospital day 9. On hospital day 10, he was transferred from the ICU to the general ward on high flow nasal cannula with a FiO2 of 0.5. The timeline of the major events and treatment was shown in the Fig. 3. As his PaO2/FiO2 ratio improved, the methylprednisolone (60 mg) was switched to prednisolone (60 mg). The histopathologic findings of the open lung biopsy were as follows: DAH, organization with accumulation of hemosiderin-laden macrophages and a few fibroblastic foci, and hyperplasia of type II pneumocytes (Fig. 4). The histology revealed no evidence of vasculitis, pulmonary veno-occlusive disease, or HP such as granulomatous inflammation of alveoli and interstitial tissue. A thorough evaluation revealed no evidence of the other known causes of DAH (connective tissue disease, systemic vasculitis, drugs, or infections). He remained in the hospital for an additional 2 weeks due to subcutaneous emphysema. At discharge, he was taking prednisolone (60 mg), did not require supplemental oxygen, and had a near normal chest radiography on hospital day 24 (Fig. 5). Currently, he continues to take prednisolone and the dosage is being gradually tapered in the outpatient clinic.
Exposure to organic dust can cause various kinds of pulmonary disease including HP, organic dust toxicity syndrome (ODTS), and bronchitis.[2,3] However, the association of DAH and exposure to organic dust is not well known. There are only one report of a patient with DAH caused by exposure to organic dust, however, the clinical presentation and image findings were different from this case.[4] To the best of our knowledge, this is the first case report of histopathologically confirmed DAH associated with exposure to organic particles.
HP should be considered first for these kinds of pulmonary conditions caused by exposure to a variety of organic particles. In particular, subacute HP should have been considered as a differential diagnosis for this case since it can result from repeated low-level exposure to inhaled organic antigen. Subacute HP is usually progressive and presents with persistent cough and dyspnea.[2] The radiologic findings are quiet variable, including ground-glass opacities or poorly defined small nodules found by a chest CT.[2] BAL fluid from patients with HP typically shows neutrophilia and lymphocytosis with a decreased CD4/CD8 ratio (<1). This is distinct from DAH in this case. Furthermore, the pathologic findings in this case revealed no evidence of HP.
ODTS is a self-limited syndrome characterized by flu-like systemic symptoms including fever, chills, myalgia, dyspnea, and cough following respiratory exposure to organic particles.[3,5] This syndrome usually begins within hours of an exposure to substantial organic particles, which is similar to acute HP. However, chest radiograph in ODTS is usually normal,[3,5] meanwhile those in HP are not normal as previously described.[2] In addition, ODTS can be distinguished from HP by its self-limiting property and a patient’s normal level of oxygen saturation.
DAH refers to a significant condition that involves bleeding into the alveolar space caused by various conditions. The mechanism of DAH after exposure to organic dust in this case was uncertain, however, activation of pulmonary macrophages and release of variable mediators and complement system might be related to the reaction after inhalation of organic dust.[6] BAL is an important tool for the diagnosis of DAH, which causes progressively bloodier fluid from subsequent aliquots. The radiological findings of DAH depend on its clinical course and can include findings such as patchy ground-glass opacities, diffuse centrilobular ground-glass opacities, interstitial thickening, and fibrosis. In many cases, it is difficult to distinguish DAH from HP by only using findings on imaging. Treatment of DAH is based on the underlying etiology and usually includes corticosteroids and other immunosuppressants. In this case, we used high-dose corticosteroid for possible etiologic mechanisms including immune response and severe inflammation after ruling out infectious conditions. In idiopathic DAH, high-dose corticosteroid and azathioprine might be considered.[7]
In conclusion, this is the first case of severe DAH associated with exposure to an organic particle to be histopathologically confirmed. Although the development of DAH associated with respiratory exposure to organic dust has rarely been reported in literature, it might be considered in the differential diagnosis for cases presenting with hemoptysis and respiratory failure following repeated exposure to organic particles. In these cases, high-dose corticosteroids might be considered for empirical therapy after infectious conditions have been excluded.
References
2. Selman M, Pardo A, King TE Jr. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012; 186:314–24.
3. Seifert SA, Von Essen S, Jacobitz K, Crouch R, Lintner CP. Organic dust toxic syndrome: a review. J Toxicol Clin Toxicol. 2003; 41:185–93.

4. Suzuki Y, Imokawa S, Nihashi F, Uto T, Sato J, Suda T. Diffuse alveolar hemorrhage caused by exposure to organic dust. Respir Med Case Rep. 2015; 15:59–61.

5. Blanc PD. Husker days and fever nights: counting cases of organic dust toxic syndrome. Chest. 1999; 116:1157–8.
6. Christiani DC. Organic dust toxic syndrome. Preventing occupational disease and injury. 2nd ed. In : Levy BS, Wagner GR, Rest KM, Weeks JK, editors. Washington, DC: American Public Health Association;2005. –368.
Fig. 1.
Initial chest radiographic findings. (A) Initial chest radiograph of the patient showing diffuse bilateral infiltration. (B) Chest computed tomography showing diffuse ground glass opacities and consolidation with centrilobular pattern involving both lungs suggesting diffuse alveolar hemorrhage or acute hypersensitivity pneumonitis.
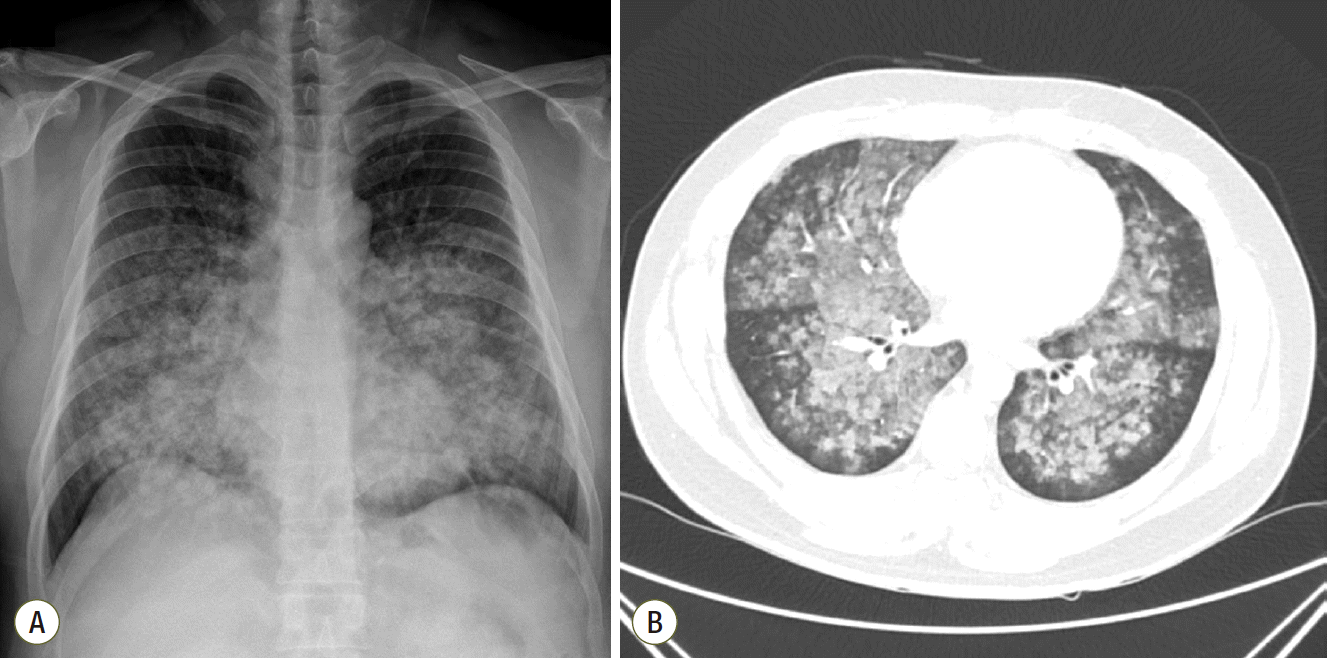
Fig. 2.
Result of bronchoalveolar lavage. Bronchoalveolar lavage showing typical progressively bloody returns in the sequential aliquots.
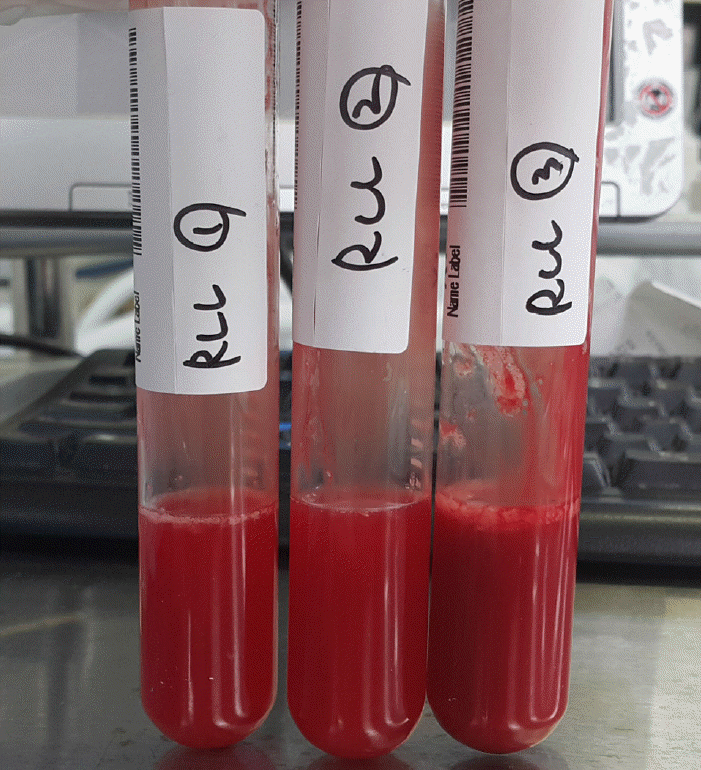
Fig. 3.
Summary of key events and the treatment. MPD: methylprednisolone; NO: nitrous oxide; HD: hospital day; ED: emergency department; EICU: emergency intensive care unit; MV: mechanical ventilation.
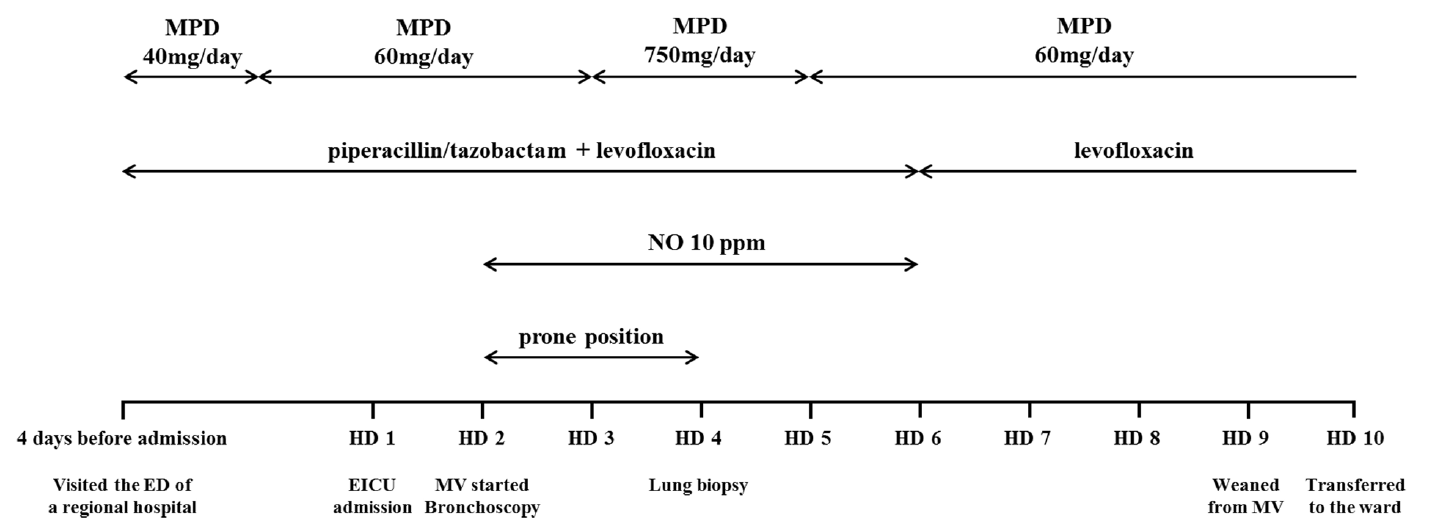
Fig. 4.
Histopatholgic findings of open lung biopsy. (A) Diffuse accumulation of red blood cells in the alveolar spaces and interstitial thickening are observed, consistent with diffuse alveolar hemorrhage and organization (hematoxylin and eosin staining (H&E), x100). (B) Some fibroblastic foci are noted in the specimen (H&E, x200). (C) Numerous hemosiderin-laden macrophages and hyperplasia of type II pneumocytes are observed, representing alveolar hemorrhage (H&E, ×400).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download