Abstract
Pnuemocystis jirovecii pneumonia (PJP) is one of leading causes of acute respiratory failure in patients infected with human immunodeficiency virus (HIV), and the mortality rate remains high in mechanically ventilated HIV patients with PJP. There are several reported cases who received extracorporeal membrane oxygenation (ECMO) treatment for respiratory failure associated with severe PJP in HIV-infected patients. We report a patient who was newly diagnosed with HIV and PJP whose condition worsened after highly active antiretroviral therapy (HAART) initiation and progressed to acute respiratory distress syndrome requiring veno-venous ECMO. The patient recovered from PJP and is undergoing treatment with HAART. ECMO support can be an effective life-saving salvage therapy for acute respiratory failure refractory to mechanical ventilation following HAART in HIV-infected patients with severe PJP.
Extracorporeal membrane oxygenation (ECMO) is an effective life-saving support therapy for patients with acute respiratory distress syndrome (ARDS) who are refractory to maximal mechanical ventilation.[1] A cohort study in which patients with severe ARDS were included during the 2009 influenza A (H1N1) pandemic as well as a randomized controlled trial in which patients with severe but potentially reversible respiratory failure were enrolled showed the clinical efficacy of ECMO. [2,3] Pnuemocystis jirovecii pneumonia (PJP) is one of leading causes for acute respiratory failure among patients with human immunodeficiency virus (HIV) infection admitted to intensive care units.[4] Chemoprophylaxis regimen and highly active antiretroviral therapy (HAART) for PJP have reduced the morbidity and mortality among HIV-infected patients.[5] However, severe cases of PJP requiring mechanical ventilation have high rates of mortality.[6,7] A few HIV patients who received ECMO as salvage therapy for respiratory failure associated with severe PJP have been reported.[8-11] Among them, the use of ECMO following the worsening of clinical conditions after initiation of HAART has rarely been described.[8,10] Here, we report a case of severe PJP in a newly diagnosed HIV patient who experienced clinical deterioration following HAART initiation and developed ARDS, necessitating veno-venous ECMO. We also review similar cases reported in the literature.
A 59-year-old Korean man was admitted to a local general hospital with a 4-day history of fever, dry cough, and progressive dyspnea on exertion. Chest radiography on admission showed bilateral patchy increased radiopacity (Fig. 1A), and chest computed tomography revealed diffuse ground-glass opacities with a mosaic pattern (Fig. 1B). Because rapid HIV antibody testing was positive, pneumonia caused by an atypical pathogen such as Pneumocystis jirovecii or cytomegalovirus (CMV) was suspected, and the patient was transferred to our institution. He had lost 10 kg body weight during the previous year and had felt sick for a month. He was a former 20-pack-year smoker with heavy alcohol consumption (more than three alcoholic beverages per week). He had had sexual encounters with men during his 50s. On arrival, he was conscious but appeared acutely ill. Blood pressure was 110/70 mmHg, heart rate 116 beats/min, respiratory rate 26 breaths/min, and body temperature 38.8°C. The initial laboratory evaluation revealed a white blood cells were 7,300/mm3 (neutrophils, 70.9%; lymphocytes, 17.6%; and eosinophils, 0.3%); hemoglobin, 11.9 g/dL; platelets, 101,000/mm3; C-reactive protein was 8.13 mg/L (reference range, 0−0.3 mg/L); procalcitonin 0.07 ng/mL (reference range, 0−0.05 ng/mL). Laboratory findings including liver and renal function tests were within normal limits. His initial arterial blood gas showed hypoxemia (pH = 7.543, pCO2 = 29.2 mmHg, PaO2 = 53.8 mmHg, HCO3 = 25.1 mmol/L, and SaO2 = 88.1% in room air). Oral trimethoprim/sulfamethoxazole (TMP/SMX) and intravenous gancyclovir were initiated on admission. On the second day of admission, bronchoscopy with bronchoalveolar lavage (BAL) demonstrated organisms consistent with Pneumocystis jirovecii, for which oral TMP/SMX was continued with corticosteroids. CMV infection was excluded due to negative results on CMV antigenemia and culture of BAL fluid, for which gancyclovir therapy was withdrawn on the third day of admission. Other bacterial, viral, and fungal infections were excluded by appropriate microscopic, culture-based, or molecular methods using BAL. Polymerase chain reaction analysis of BAL fluid was positive for PJP. The peripheral HIV viral load was 442,000 copies/mL, and the total CD4 T-cell count was 89 cells/μL3 (CD4% = 7.0%). After 6 days of TMP/SMX with corticosteroids, his symptoms and chest radiography findings improved (Fig. 1C). HAART using tenofovir, emtricitabine, and efavirenz was subsequently started on hospital day 7. His therapy for PJP was then changed to clindamycin and primaquine due to severe nausea caused by the TMP/SMX with gradual tapering of adjunct corticosteroids.
However, his respiratory status deteriorated with worsening hypoxemia. Nine days after initiation of HAART, he developed ARDS requiring intubation and mechanical ventilation (Fig. 1D and E). Despite the use of all available mechanical ventilator support at this institution (conventional lung-protective ventilation and neuromuscular blocking), arterial blood gas analysis showed refractory respiratory acidosis and hypoxemia (pH 7.09, PaCO2 = 102.0 mmHg, PaO2 = 85 mmHg, HCO3 = 30.9 mmol/L, and SaO2 = 92% on 100% fraction of inspired oxygen). The patient was administered peripheral, percutaneous, right femoral vein-right internal jugular vein ECMO support on hospital day 19 using the Capiox emergent bypass system (EBS; Terumo Inc., Tokyo, Japan). The respiratory ECMO survival prediction (RESP) score of this patient was -2 (immunocompromised adult aged 59, about 72 h of mechanical ventilation before ECMO, other acute respiratory diagnosis, neuromuscular blockade before ECMO, PaCO2 ≥ 75 mmHg).[12] On BAL fluid analysis, organisms consistent with PJP were still observed, albeit in much smaller numbers. We decided to reinstitute intravenous TMP/SMX therapy for PJP considering the timing of respiratory decompensation and the change in therapy to clindamycin and primaquine. Moreover, due to concern for immune reconstitution inflammatory syndrome (IRIS), corticosteroid dosing was increased to approximately 1.5 mg/kg per day with continuation of HAART. Despite the absence of documented bacterial infection, the patient was treated with piperacillin/tazobactam, which did not improve his condition. We performed CMV IgM, IgG, and antigenemia on blood to exclude the possibility of developing CMV pneumonia. The results for CMV infection were as follows: negative CMV IgM, positive CMV IgG, and low level of CMV antigenemia (16 cells/2 × 106 granulocytes); therefore, gancyclovir was not administered to the patient. All tests for other infectious agents including viral and fungal microorganisms in respiratory, blood, and urinary specimens were negative. After 5 days on ECMO, as his oxygenation improved, he was decannulated successfully. The absolute number of CD4 T-cells was 52 cells/μL3 (CD4% = 17.0%), and the plasma viral load was 586 copies/mL decreasing satisfactorily within 1 month of HAART. PJP therapy with intravenous TMP/SMX for 16 days gradually improved his clinical symptoms and chest radiograph. Although the impact of TMP-SMX resistance of Pneumocystis jirovecii was uncertain in our case, we changed TMP-SMX to primaquine and clindamycin on hospital day 32 for residual ground-glass opacity on chest radiograph and used it for another 2 weeks, which resulted in clinical and radiographic improvement. The patient’s clinical course is summarized in Fig. 2.
With mechanical ventilator treatment after ECMO, the patient’s clinical course was further complicated by the development of drug resistant hospital-acquired infections. He was treated with vancomycin and imipenem/cilastatin due to culture for arterial line of methicillin-resistant coagulase-negative staphylococci and extended-spectrum β-lactamase-positive Klebsiella pneumonia. Along with progression of multifocal infiltrations in both lungs, repeated cultures of his tracheal aspiration revealed carbapenem-resistant Acinetobacter baumanii. On hospital day 32, the antibiotics were changed from imipenem/cilastatin to tigecycline and nebulized colistin. We treated the patient with a 14-d course of gancyclovir due to increasing CMV antigenemia (273 cells/2 × 106 granulocytes) on hospital day 34. He was successfully weaned from the mechanical ventilator on hospital day 42, and discharged on hospital day 122.
Cases of ECMO support for refractory respiratory failure in HIV-infected patients during PJP treatment have rarely been reported in the literature.[8-10] The characteristics of the patients including our case are presented in Table 1. All cases had a baseline CD4 T-cell count of less than 100 cells/μL3, susceptible to opportunistic infection, and were unaware of their HIV diagnosis or were not receiving HIV treatment at the time of diagnosis of PJP. They had severe hypoxemia associated with PJP, requiring ECMO support. Interestingly, only three cases including ours suffered from paradoxical worsening that necessitated ECMO after starting HAART.[8,10] To our knowledge, our case is the first in South Korea of ECMO use for ARDS after HAART introduction in an HIV-infected patient being treated for PJP.
Patients recently started on HAART may experience paradoxical deterioration in their symptoms and respiratory status. This phenomenon, which is known as IRIS, is a consequence of the recovery of immune function after initiation of HAART.[13] IRIS has been reported in patients with PJP starting HAART; however, it has only rarely been life-threatening in this situation.[14,15] PJP and Pneumocystis jirovecii-related IRIS contributed to acute respiratory failure requiring ECMO support in two previous cases (patients 2[8] and 3[10]). They had severe PJP that led to the diagnosis of HIV, which clinically improved with PJP treatment and adjunctive corticosteroids. Our case had similar features of severe hypoxemia and ARDS, which improved on ECMO support with an increase in corticosteroid dose. The clinical course of our case was consistent with that of IRIS. Other conditions, including pulmonary co-infection by other organisms, progressive disease due to poor compliance, and Pneumocystis organisms’ potential resistance to TMP/SMX were also considered.[16,17] No bacterial, viral, or fungal pathogens were found in the blood, urine, or BAL fluid in repeated examinations, with the exception of Pneumocystis jirovecii in BAL fluid. Moreover, the plasma HIV viral load decreased significantly following HAART introduction within 1 month, showing that HAART was successful. Notably, TMP/SMX was used in our case, resulting in significant improvement.
It is interesting to note that in all three cases, HAART was initiated within 2 weeks of PJP therapy. The optimal timing of HAART in patients with HIV and PJP has yet to be determined, because HAART may affect the host immune response, which can lead to a variety of adverse clinical manifestations, especially IRIS.[13,16] There are two clinical strategies regarding initiation of HAART: ‘early HAART’, intended to be initiated during treatment of PJP, and ‘deferred HAART’, intended to be initiated after treatment of PJP. A recent randomized controlled trial in which 282 HIV-infected patients with opportunistic infections participated, 63% of whom had PJP, found that IRIS developed a median of 33 days after initiation of HAART. The study reported favorable results of early HAART (median 12 days), showing a significantly lower incidence of HIV progression/death and no increase in adverse events, compared with deferred initiation of HAART (median 45 days).[15] Thus, US guidelines recommend that HAART be started within 2 weeks of the diagnosis of PJP in a HAART naïve population, when possible.[18] However, all three cases suggest that we need to be mindful of the increasing risks of IRIS in those who have early HAART initiation. Moreover, the time to initiation of ECMO in these cases was approximately 2 weeks after HAART initiation, which was shorter than previous results. While ECMO support in the two previous cases[8,10] did not lead to patient survival, this case demonstrated successful weaning off of treatment and a favorable outcome (18 and 12 days, respectively, Table 1). These findings suggest that early clinical suspicion of Pneumocystis jirovecii-related IRIS and adequate therapy are required for better outcomes in such cases.
Despite the relevance of immune reconstitution in our case, the possibility of insufficient treatment for PJP might have affected the paradoxical deterioration. TMP/SMX is the most frequently used first-line treatment agent for PJP in HIV-infected patients, and intravenous therapy is generally recommended for those with moderate to severe PJP. [17] As an alternative regimen, clindamycin and primaquine can be selected for patients who fail to respond to or develop toxicity with TMP/SMX.[19] Due to severe nausea to oral and intravenous TMP/SMX, our patient’s PJP therapy was changed to clindamycin and primaquine at the time of HAART administration. Paradoxical deterioration with ARDS improved with the change in PJP therapy to intravenous TMP/SMX, as well as ECMO support. Similarly, patient 3[10] was started on intravenous pentamidine as a first-line regimen due to reported allergy to TMP/SMX. Shortly before ECMO initiation, intravenous TMP/SMX was administered through a desensitization procedure. Treatment failure with the accepted regimen is uncommon; however, there was indeed a temporal connection between respiratory decompensation and alteration of the therapeutic regimen for PJP, as well as HAART initiation.
This case report provides an opportunity to address the use of ECMO in patients with HIV infection. ECMO is usually not recommended in patients with significant preexisting comorbidities or those who are immunocompromised due to poor outcomes.[20] A high mortality rate has been reported in mechanically ventilated HIV-infected patients with severe PJP,[6,7] for whom ECMO support may seem to be medically futile. The RESP score was developed to predict survival after ECMO initiation from patients receiving ECMO for severe acute respiratory failure and comprised 12 simple preECMO variables including immunocompromised status. [12] The RESP score of our case was -2, correlating with a survival of only 33% and ARDS presentation requiring ECMO in a HIV-infected patient was previously considered less likely to benefit from ECMO. However, he improved clinically with ECMO support and survived to hospital discharge. Thus, ECMO support may be an effective salvage therapy in severe respiratory failure following HAART initiation in HIV-infected patients with PJP.
In conclusion, several conditions may cause paradoxical deterioration in adult HIV-infected patients who are undergoing treatment for severe PJP. The clinical presentation of our case was consistent with IRIS in HIV patients during treatment of PJP; however, the clinical manifestations could be alternatively explained by insufficient treatment for PJP. Despite a very small number of cases in the literature, we experienced ARDS as a paradoxical sign of worsening following early HAART introduction in an HIV patient, and ECMO support may be beneficial in this situation.
References
1. Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. 2011; 365:1905–14.

2. Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011; 306:1659–68.

3. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009; 374:1351–63.

4. Barbier F, Coquet I, Legriel S, Pavie J, Darmon M, Mayaux J, et al. Etiologies and outcome of acute respiratory failure in HIV-infected patients. Intensive Care Med. 2009; 35:1678–86.

5. Kelley CF, Checkley W, Mannino DM, Franco-Paredes C, Del Rio C, Holguin F. Trends in hospitalizations for AIDS-associated Pneumocystis jirovecii Pneumonia in the United States (1986 to 2005). Chest. 2009; 136:190–7.

6. Khouli H, Afrasiabi A, Shibli M, Hajal R, Barrett CR, Homel P. Outcome of critically ill human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. J Intensive Care Med. 2005; 20:327–33.

7. Morris A, Wachter RM, Luce J, Turner J, Huang L. Improved survival with highly active antiretroviral therapy in HIV-infected patients with severe Pneumocystis carinii pneumonia. AIDS. 2003; 17:73–80.

8. Cawcutt K, Gallo De Moraes A, Lee SJ, Park JG, Schears GJ, Nemergut ME. The use of ECMO in HIV/AIDS with Pneumocystis jirovecii Pneumonia: a case report and review of the literature. ASAIO J. 2014; 60:606–8.
9. De Rosa FG, Fanelli V, Corcione S, Urbino R, Bonetto C, Ricci D, et al. Extra Corporeal Membrane Oxygenation (ECMO) in three HIV-positive patients with acute respiratory distress syndrome. BMC Anesthesiol. 2014; 14:37.

10. Goodman JJ, Goodman LF, Sarvepalli SK, Firstenberg MS, Lustberg ME, Bazan JA. Extracorporeal Membrane Oxygenation as Adjunctive Therapy for Refractory Hypoxemic Respiratory Failure in HIV-positive Patients With Severe Pneumocystis jirovecii Pneumonia. Clin Pulm Med. 2013; 20:117–20.

11. Gutermann H, van Roy B, Meersseman W, Meyns B, Herijgers P. Successful extracorporeal lung assistance for overwhelming pneumonia in a patient with undiagnosed full blown aids--a controversial therapy in HIV-patients. Thorac Cardiovasc Surg. 2005; 53:252–4.
12. Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014; 189:1374–82.

13. Barry SM, Lipman MC, Deery AR, Johnson MA, Janossy G. Immune reconstitution pneumonitis following Pneumocystis carinii pneumonia in HIV-infected subjects. HIV Med. 2002; 3:207–11.

14. Wislez M, Bergot E, Antoine M, Parrot A, Carette MF, Mayaud C, et al. Acute respiratory failure following HAART introduction in patients treated for Pneumocystis carinii pneumonia. Am J Respir Crit Care Med. 2001; 164:847–51.
15. Zolopa A, Andersen J, Powderly W, Sanchez A, Sanne I, Suckow C, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One. 2009; 4:e5575.

16. Müller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M, et al. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010; 10:251–61.

17. Huang L, Cattamanchi A, Davis JL, den Boon S, Kovacs J, Meshnick S, et al. HIV-associated Pneumocystis pneumonia. Proc Am Thorac Soc. 2011; 8:294–300.

18. National Institutes of Health; AIDSinfo. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. 2016. May. [2016 May]. Available from https://aidsinfo.nih.gov/guidelines/html/4/adult-and-adolescent-oi-prevention-andtreatment-guidelines/0.
Fig. 1.
Chest radiograph and computed tomography. Chest radiography and computed tomography images were obtained on hospital days 1 (A, B), 6 (C), and 19 (D, E). A and B. Chest radiographs show increased patchy opacities, and chest computed tomography reveals diffuse ground-glass opacities with a mosaic pattern in both lungs on admission. C. Chest radiography reveals mild improvement of the ground-glass density in both lungs after TMP/SMX therapy with corticosteroids on hospital day 6. D and E. Aggravated diffuse consolidation and ground-glass opacities were noted on chest radiograph and computed tomography on hospital day 19 at the time of ECMO initiation. ECMO: extracorporeal membrane oxygenation; TMP/SMX: trimethoprim/sulfamethoxazole.
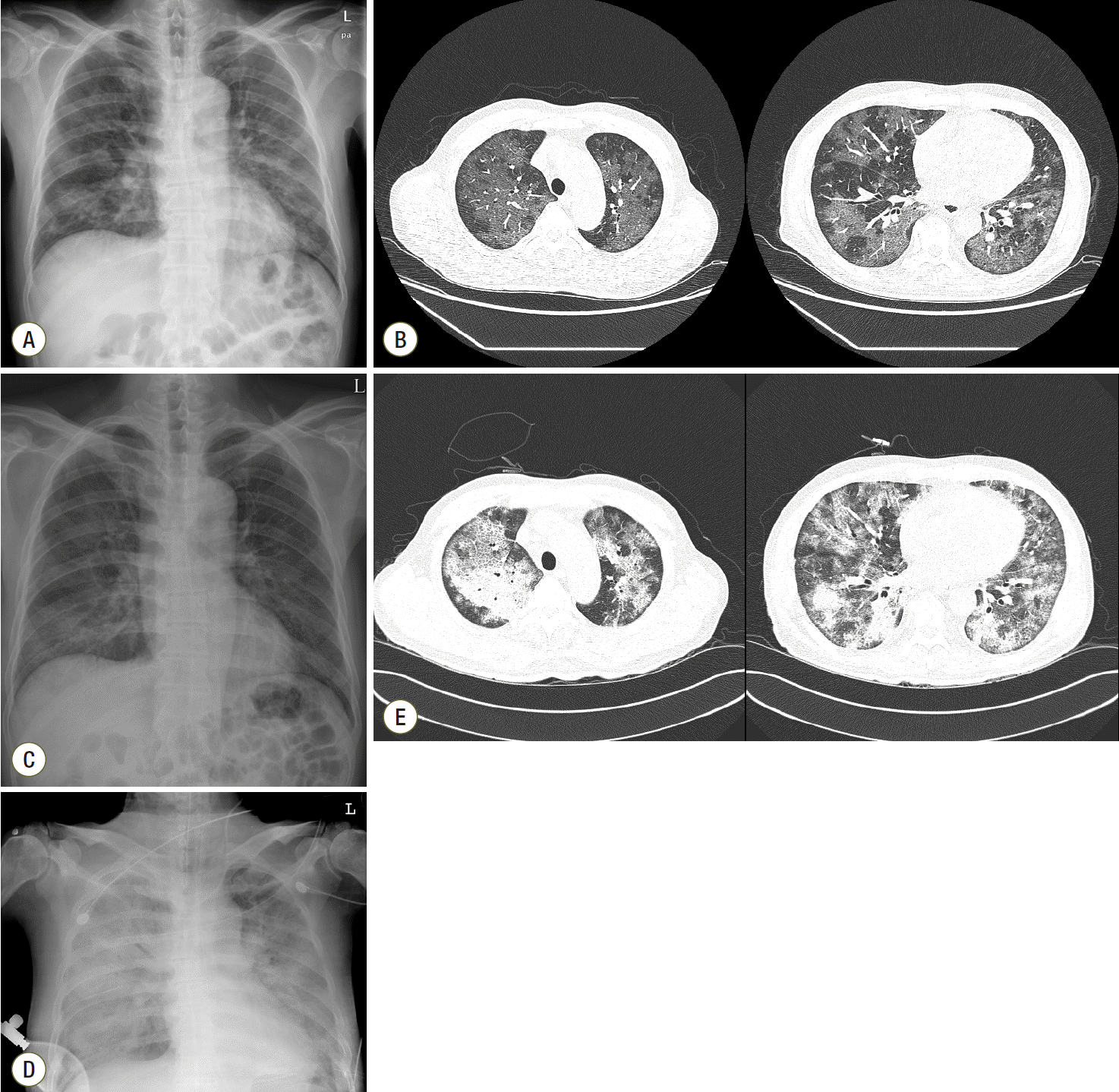
Fig. 2.
Sequence of major clinical and laboratory events. HD: hospital days; TMP/SMX: trimethoprim/sulfamethoxazole; IV: intravenous; HAART: highly active antiretroviral therapy; MV: mechanical ventilation; ECMO: extracorporeal membrane oxygenation; PJP: Pnuemocystis jirovecii pneumonia; BAL: bronchoalveolar lavage; PCR: polymerase chain reaction; HIV: human immunodeficiency virus.
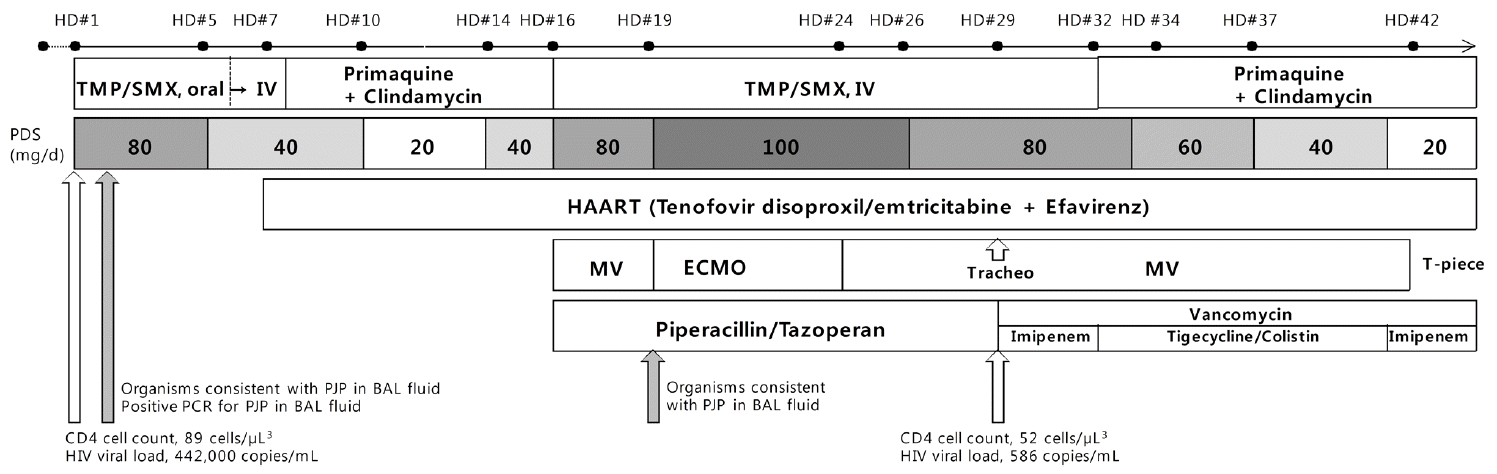
Table 1.
Clinical characteristics of HIV-infected adult patients with severe Pneumocystis jirovecii pneumonia supported by ECMO
| Patient (Ref.) country, yr | Age (y)/sex | Detection of HIV | CD4 (cells/μL)/CD4% (%)/viral load (copies/mL) at diagnosis | PaO2 (mmHg)/FiO2 (%)/P/F ratio | Anti-PJP therapy |
Timing of HAART initiation |
ECMO initiation (HD)/ECMO duration (days) | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| Pre-, On-, Post-ECMO | Days from anti-PJP therapy | ||||||||
| 1. This case South Korea, 2015 | 59/M | At diagnosis | 89/7/442,000 | 78/100/78 | TMP/SMX→CLI+PQ→TMP/SMX→CLI+PQ | Pre* | 7 | 19/5 | Survived to hospital discharge |
| 2. Cawcutt et al. [8] US, 2014 | 45/M | At diagnosis | 33/NR/113,000 | 59/60/98 | TMP/SMX→CLI+PQ | Pre* | Subsequently started | 5(18‡)/57 | Success of decannulation Died in hospital |
| 3. Goodman et al. [10] US, 2013 | 25/M | At diagnosis | 36/17/622,234 | 63.6/100/63.6 | Pentamidine→CLI+PQ→TMP/SMX | Pre* | Subsequently started | 18/69 | Died on ECMO |
| 4. De Rosa, et al. [9] Italy, 2014 | 21/F | Congenital HIV, 2-yr HAART discontinuation | 2/NR/118,330 | NR/NR/120 | TMP/SMX→CLI+PQ | NR* | NA | 8/20 | Survived to hospital discharge |
| 5. De Rosa, et al. [9] Italy, 2014 | 24/M | At diagnosis | 3/NR/50,728 | NR/NR/100 | TMP/SMX→CLI+PQ+Caspofungin | Post* | NA | 10/24 | Success of decannulation Died in hospital |
| 6. Goodman et al. [10] US, 2013 | 30/F | At diagnosis | 13/4.5/976,631 | 50.1/100/50.1 | TMP/SMX | Post* | NA | 3/7 | Survived to hospital discharge |
| 7. Gutermann et al. [11] Belgium, 2005 | 55/M | At diagnosis | 9/2.9/80,235 | NR/NR/NR | TMP/SMX | Post† | NA | 4/4 | Survived to hospital discharge |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download