Invasive aspergillosis (IA) is most commonly seen in patients with risk factors who are undergoing cytotoxic chemotherapy, have prolonged neutropenia, are taking corticosteroids, have undergone transplant, and have acquired immune deficiency syndrome (AIDS).[
1] However, there are a growing number of reports of IA cases among patients who do not have an immune disorder; these cases are mostly among intensive care unit (ICU) patients.[
2,
3] It is difficult to diagnose IA in immunocompetent patients during its early septic period. Consequently, diagnosis and treatment of IA is often delayed, decreasing the likelihood of a positive outcome.[
3] Moreover, in patients with extra-pulmonary IA without dissemination, it is especially difficult to diagnosis. This report presents a case of isolated invasive intestinal aspergillosis developed without dissemination in a patient with no classic predisposing risk factors.
Case Report
A 53-year-old male was referred to our emergency department in a state of shock after drinking a large amount of alcohol over a period of a week. He was a heavy drinker (1 bottle of Soju/day for the past 5 years) and 21 years ago he underwent subtotal gastrectomy to treat a perforated gastric ulcer. He had no diseases that required habitual medication.
At presentation, the patient’s blood pressure was 82/50 mmHg; his pulse rate was 78 beats per minute; his respiratory rate was 28 breaths per minute; and his body temperature was 34ºC. We conducted a limited physical examination because of his drowsy mental state, but was at least able to note the patient’s distended abdomen without muscle guarding. Arterial blood gas analysis revealed that the patient had severe metabolic acidosis (pH 6.74, PCO
2 21.1 mmHg, PaO
2 100.9 mmHg, HCO
3- 2.8 mmol/l, lactic acid 17.55 mmol/l). The laboratory test results of this patient were as flows: hemoglobin: 10.1 g/dL, total leukocyte count: 23,060 cells/μL (segment neutrophils: 90%), platelet count: 23,000 cells/μL, blood urea nitrogen: 20.9 mg/dL, creatinine: 2.08 mg/dL, total bilirubin: 1.8 mg/dL, C-reactive protein: 42.75 mg/dL, and procalcitonin: 158.22 ng/mL. Initial abdominopelvic computed tomography (APCT) showed acute pancreatitis with peripancreatic infiltration and severe enterocolitis (
Fig. 1A and
1B). He was immediately transferred to the medical ICU for management of his septic shock with multi-organ failure. Aggressive resuscitation and antibiotics (piperacillin/tazobactam at 2.25 g, every 6 hours) were initiated and the patient was treated with a mechanical ventilator and continuous renal replacement therapy (CRRT). He required high-dose vasopressors (1.43 μg/kg/min of norepinephrine, 0.04 IU/min of vasopressin, and 0.5 μg/kg/min of epinephrine), so we started him on 200 mg/day of hydrocortisone. There was no growth of blood cultures.
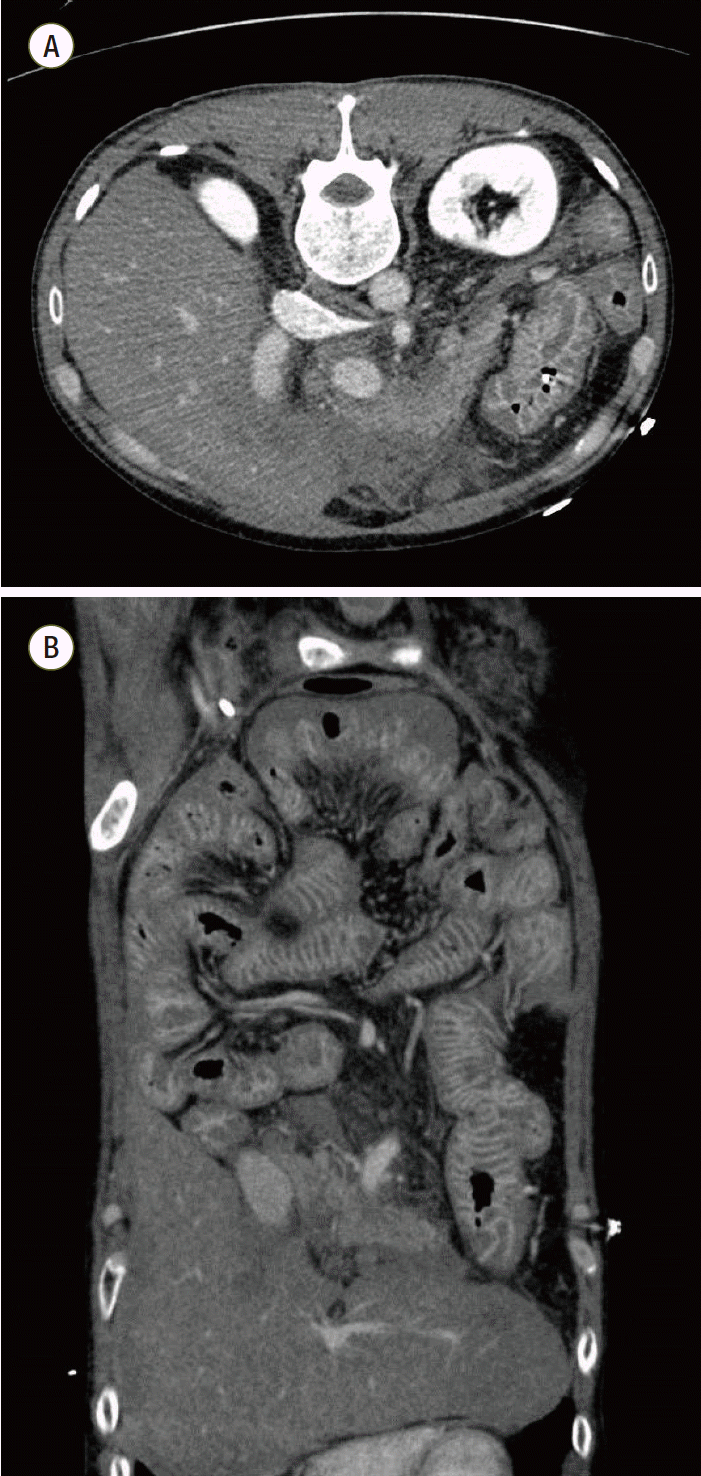 | Fig. 1.Abdominopelvic computed tomography shows acute pancreatitis with peripancreatic infiltration and severe enterocolitis. (A) Transverse computed tomography (CT) image (B) Coronal CT image. Hospital day 1. 
|
After 5 days in the ICU, follow-up APCT showed that his acute pancreatitis was unchanged but that his enterocolitis had improved. And there were no evidence of microorganisms in follow up cultures. On his ninth day in ICU, he was weaned off the mechanical ventilator, vasopressors, CRRT and steroids. The next day, while still in the ICU, he developed hematochezia, but did not have any abdominal pain or tenderness. Sigmoidoscopic findings and biopsy indicated ischemic colitis (
Fig. 2). However, his vital sign was stable and there was no sign of peritonitis. Therefore, we managed his condition conservatively with surgical observation. On his thirteenth day in ICU, he had a second attack of septic shock, with the following vitals: blood pressure, 75/43 mmHg; pulse rate, 89 beats per minute; respiration rate, 33 breaths per minute and body temperature, 38.6ºC. His abdomen was distended and intra-abdominal pressure was 19 cmH
2O. APCT indicated he had advanced intestinal ischemia and peritonitis with no change in his pancreatitis. He was transferred to the operating room for surgical exploration and we found multifocal ischemic and necrotic changes through his whole bowel except for an approximately 100 cm segment of his proximal jejunum. We performed a near total small bowel resection, total proctocolectomy and end jejunostomy. Cultured intra-abdominal fluid produced vancomycin-sensitive enterococci and multidrug-resistant coagulase-negative staphylococci; we immediately administered the patient with vancomycin and piperacillin/tazobactam. On postoperative day (POD) 4, the patient was once again weaned off the ventilator, CRRT, and vasopressors. On POD 7, pathologic reports revealed segmental total ischemic necrosis with invasive aspergillosis. The resected specimen showed septated fungal hyphae with acute angle branching, suggesting
Aspergillus species with angioinvasion (
Fig. 3A and
3B). Unfortunately, fungal culture of the resected bowel was not available at that time. A serum
Aspergillus galactomannan (GM) antigen test was positive (Enzyme linked immunoassay, index value 3.07) and
Candida albicans was cultured from the intra-abdominal fluid, so we started liposomal amphotericin B treatment for 19 days. Additional intra-abdominal drainage catheters were inserted at POD 10 due to infected fluid collection and SMA branch embolization was done at POD 12 due to jejunostomy site bleeding. After these additional treatments, 63 days after admission to the ICU, he transferred to a local hospital. Another 2 months later, all intra-abdominal drains and the dialysis catheter were removed. However he needed parenteral nutrition support due to short bowel syndrome.
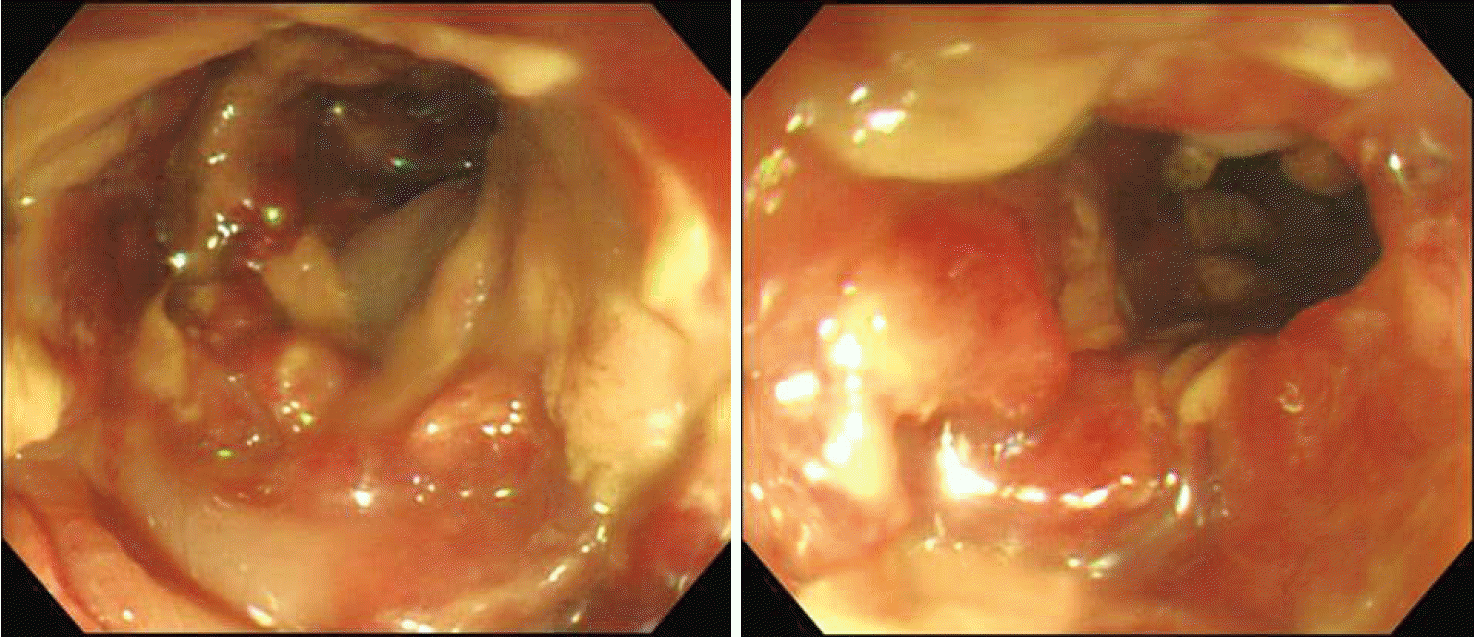 | Fig. 2.A diffuse geographically-shaped mucosal ulcer was observed beginning at the anal verge (AV) 15 to 20 cm and AV 25 cm to upward on sigmoidoscopy, which is suggestive of ischemic colitis. Biopsy was done at the AV 15cm. Hospital day 10. 
|
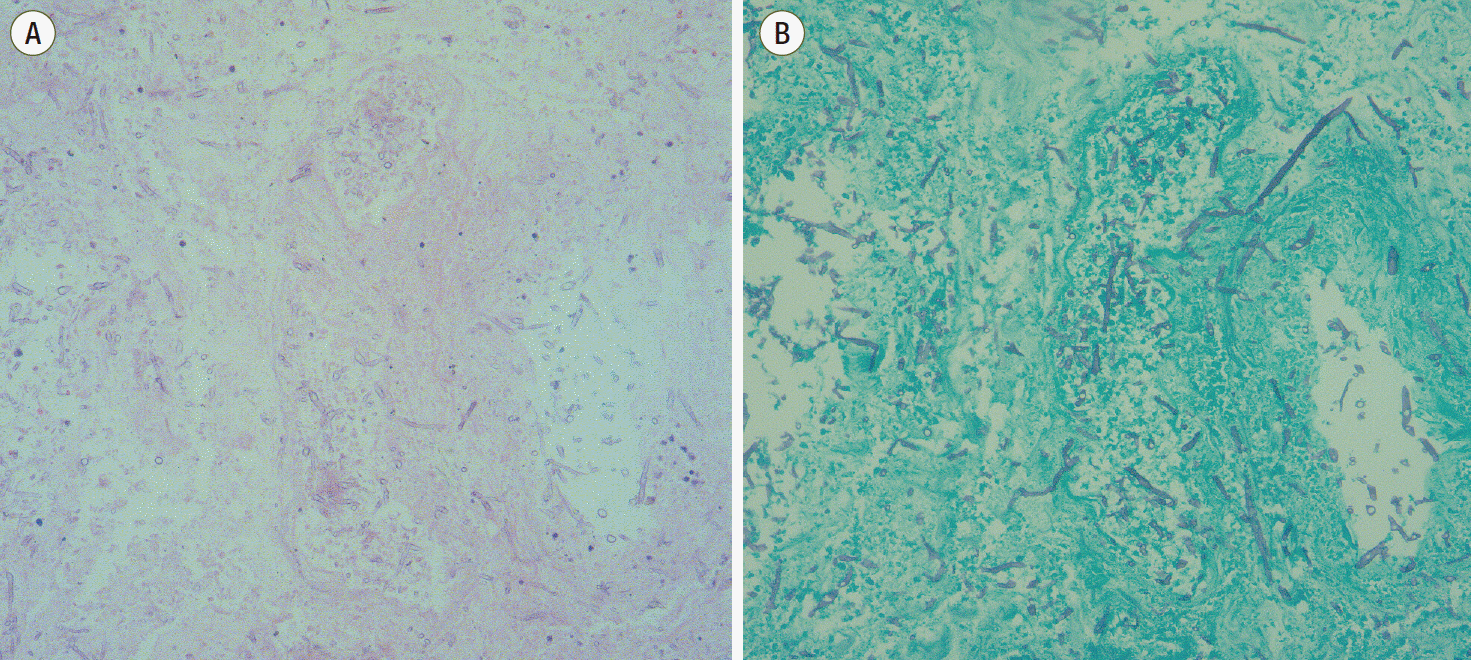 | Fig. 3.Hematoxylin and eosin (A) and Grocott’s methenamine silver (B) stains (original magnification, x200) of the resected terminal ileum: The specimen shows numerous fungal hyphae with acute angle branching and angioinvasion, which is morphologically compatible with a diagnosis of invasive aspergillosis. Pathologic result was reported at postoperative day 7 (hospital day 22). 
|
Go to :

Discussion
Risk factors for IA include undergoing cytotoxic chemotherapy, having prolonged neutropenia, taking corticosteroids, having received an organ transplant and having AIDS. Aerosolized
Aspergillus spores may become a potential source of infection in the upper respiratory tract and the alveoli manifesting as pneumonia.[
1]
Aspergillus spores can be ingested and reach the gastrointestinal (GI) tract, but they cannot penetrate a normal intact mucosal barrier. However, in pathological condition such as gastric ulcers and severe gastritis, penetration of the mucosal barrier by
Aspergillus spores is possible. In these situations, these spores can cause invasive GI aspergillosis.[
4]
There are no well-known risk factors for invasive aspergillosis among ICU patients who are not immunosuppressed. However after an extended ICU stay, hemodialysis, low-dose steroids, chronic obstructive pulmonary diseases, congestive heart failure, liver disease and diabetes mellitus (DM) could all be potential risk factors for IA.[
5] Cha et al.[
6] and Choi et al.[
7] reported invasive GI aspergillosis in immunocompetent patients. They described lower GI aspergillosis cases in non-neutropenic patients. However, in their case reports, predisposing colon cancer and DM may be associated with immune dysfunction in these patients. In our case, the patient was a heavy drinker who was otherwise relatively healthy, and he had no history of cancer or DM. However, we administered hydrocortisone for 9 days as his initial treatment. To our knowledge, there have been no reports about risk factors for GI aspergillosis. However, sepsis, ICU care, CRRT, and steroid use could all be associated with of immune dysfunction and could, therefore, be risk factors.[
8] Furthermore, history of enterocolitis and shock-induced non-occlusive bowel ischemia could instigate GI mucosal damage. Even though the patient recovered from septic shock, his GI mucosal barrier had not fully recovered. We suspect
Aspergillus spores could penetrate the GI mucosa during that period of damage, and his initial clinical manifestation of GI aspergillosis could be hematochezia.
In general, a diagnosis of invasive pulmonary aspergillosis in neutropenic patients is made on the basis of a combination of compatible clinical findings, abnormal radiologic findings, and microbiologic confirmation or on the basis of histologic proof of tissue invasion by the fungus. Among the clinical challenges in the diagnosis of IA is the absence of early signs and symptoms for defining illness, particularly among immunocompetent patients. Cultures, the gold standard for guiding choice of antimicrobial agents, are positive in less than 50% of patients and take several days for detection.[
9] Microscopic examination of clinical specimens remains a key first diagnostic test to detect fungal elements. However, stains are limited by their inability to distinguish between morphologically similar fungi.
Aspergillus is characterized by septate hyphae with acute-angle branching, a finding that may also be similar in appearance to that seen in other moulds (
Fusarium,
Penicillium,
Psuedallescheria, and
Scopulariopsis species) with differing susceptibilities to antifungal agent. GM is an
Aspergillus-specific fungal cell wall component released during
Aspergillus growth and tissue invasion.[
10] Studies of neutropenic patients have revealed high rates of sensitivity and specificity. However, the GM test is characterized by multiple false positive, making its utility in many patients uncertain. GM demonstrates cross-activity with other fungi including
Penicillium,
Botrytis, and
Paecilomyces. False positive have also been associated with antibiotics produced by
Penicillium (piperacillin/tazobactam, amoxicillin-clavulanate).[
11]
Amphotericin B has been the dominant treatment for IA for a long time. Recently, voriconazole, a derivative of fluconazole, has become the new standard of care for treating IA because it yields better outcomes. However, the mortality rate of IA exceeds 50% and is much higher in critically ill patients (> 80%).[
2] Kazan et al.[
12] reported about GI aspergillosis in immunocompromised patients. Most had non-specific clinical manifestations; abdominal pain, diarrhea, hemorrhage, intestinal obstruction and perforation. Also, 16 out of 20 patients (80%) had a positive finding GM antigenemia test. The gut seems to be an exceptional site for
Aspergillus infection even among hematology patients. From this study, we concluded that invasive GI aspergillosis is rare and extremely difficult to diagnosis without surgical intervention because of poor symptom specificity and a lack of characteristic imaging findings. Patients with abdominal symptom and a positive GM antigenemia test that cannot be explained by respiratory lesions should be suspected to have gut aspergillosis and diagnostic procedures should be performed without delay.
Recently reports about IA, particularly pulmonary aspergillosis, are increasing among immunocompetent patients many of whom are in the ICU. Delayed diagnosis of GI aspergillosis, due to its difficulty, could lead to poor outcomes for critically ill patients. This emphasize the importance of the awareness about possible IA in critically ill patients without risk factors, and readiness to proceed with appropriated diagnosis and treatment. We should not disregard positive GM antigenemia results in a critically ill patient. Consider and investigate its clinical significance, even in the absence of classic risk factors. Especially in critically ill patients with severe disrupted GI mucosal barrier, invasive GI aspergillosis should be considered.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download