Abstract
Neurological complications following liver transplantation are more common than after other organ transplants. These complications include seizure in about 8% of cases, which is associated with morbidity and mortality. Seizure should be treated immediately, and the process of differential diagnosis has to be performed appropriately in order to avoid permanent neurologic deficit. We herein report a case of status epilepticus after liver transplantation. The status epilepticus was treated promptly and the cause of seizure was assessed. The patient was discharged without any complication.
Go to : 
The high prevalence of cirrhosis from hepatitis B, and hepatocellular cancer results in a lot of hepatic failure. Liver transplantation (LT) is considered the one of the treatments for the last stage of these hepatic diseases. The number of LT is increasing remarkably and the prognosis after operation becomes better with medical development.[1] Despite this advances, neurological complications following LT occur about 15-30%,[2] and include seizure (8.2%).[3] It is associated with longer intensive care unit stays,[4] morbidity and mortality.[3] There are many reasons for seizure; neurotoxicity induced by septic and metabolic encephalopathy, immunosuppressant medications, central nervous system infections, and cerebrovascular complications.[2] However, status epilepticus is not common symptoms in patients after LT. We herein report for a case of the status epilepticus after LT.
A 59-year-old woman (height 150 cm, weight 35.8 kg) was admitted to the clinic due to recurrent hepatic encephalopathy. She had medical history of hepatitis B virus and hepatitis C virus-related liver cirrhosis. She received hepatico-jejunostomy and cholecystectomy because of intrahepatic duct stone 10 years ago. The right upper quadrant pain and fever were newly developed, so she was transferred to the regional hospital as septic cholangitis. The disease progressed to hepatic failure; she was transferred to college hospital for LT. On arrival, mental status was drowsy, and she had large amount of ascites, jaundice, and peripheral pitting edema.
After 2 days, she underwent emergency LT from brain death donor. The anesthesia time was 14 hours 55 minutes, fluid intake was 14,110 mL and the amount of transfusion was 13,800 mL; packed red blood cell 38 units, fresh frozen plasma 12 units, platelet concentrates 24 units. Intraoperative bleeding was 11,100 mL and urine output was 1,240 mL. The operation was done and she was moved to the intensive care unit (ICU).
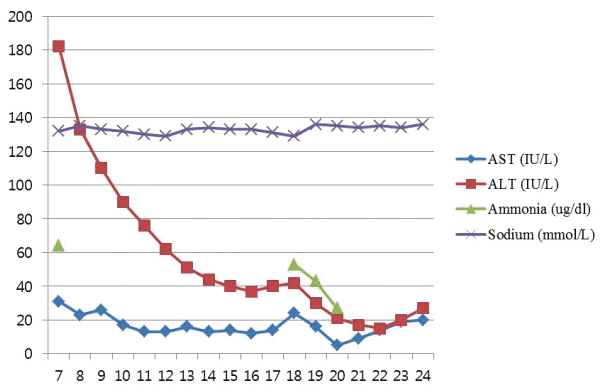
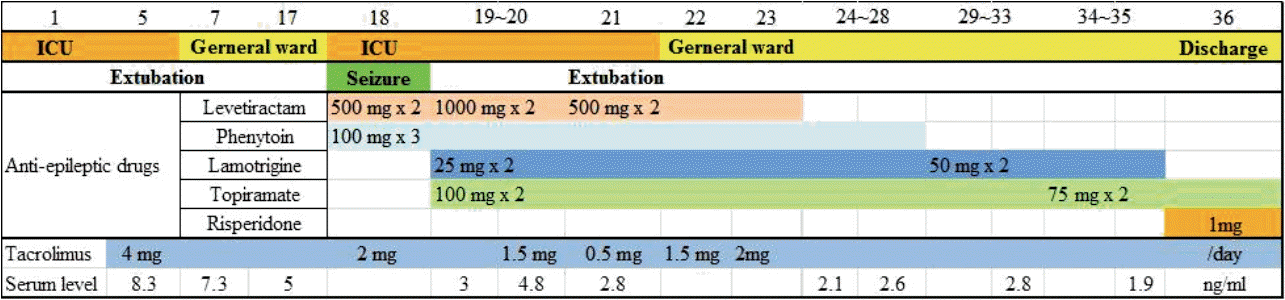
On ICU 6th day, the extubation was done, she was tolerable with 40% oxygen supply of facial mask. Her mental state was alert and oriented. On ICU 7th day, the patient was transferred to general ward. In the general ward, she spoke nonsense intermittently. After 11 days (postoperative day [POD] #17), she suffered with insomnia and delirium. The next day (POD #18), she showed the four times seizure for 10 minutes; the duration of each seizure was about 1 minute. The aura was weird voice, she presented left eye ball deviation, right arm flexion, both arm and leg synchronized jerky movement during seizure events and she continued the confused state after seizure. For treatment, lorazepam 4mg was injected and levetiracetam 1,500 mg was infused. Thereafter she was treated by levetiracetam 500 mg twice a day and phenytoin 100 mg three times a day. Her body temperature was 38.3℃. The blood pressure was 150/90 mmHg the day before the events, but it rose to 203/123 mmHg. The abnormal laboratory data were leukocytosis (white blood cells 23,410/uL, neutrophil 95.5%), and hyperglycemia (250 mg/dL). There was no electrolyte imbalance except for hyponatremia (129 mmol/L) (Fig. 1). The serum level of ionized calcium and magnesium were within normal limits during hospital days. The brain computed tomography was performed, but there was no specific finding without mild brain edema. Medical team performed the blood culture, but there was no evidence for any infection. The concentration of tacrolimus was 5 ng/mL (Fig. 2). Then, neurologist supposed to perform the cerebrospinal fluid (CSF) test. However, the electrocephalography (EEG) showed non-convulsive status epilepticus.
She was readmitted to the ICU and intubation was done. Video-EEG was monitored continuously. The antiepileptic drugs were also administered continuously (Fig. 2). Interictal EEG showed periodic spikes- or polyspikes- and slow wave complexes in right temporo-occipital region. Initially, frequent (20/hour) episodes of ictal discharges in right posterior head region, lasting about 20 to 40 seconds were shown. These discharges were occasionally accompanied by some paroxysmal, non-sterotyped movements in the right arm and leg. After high dose of midazolam (0.45-1.04 mg/kg/h) infusion, ictal discharges disappeared from second day of the treatment. Periodic epileptiform discharges also decreased, and finally, EEG showed diffuse delta and theta background slowing intermixed fast frequency activity.
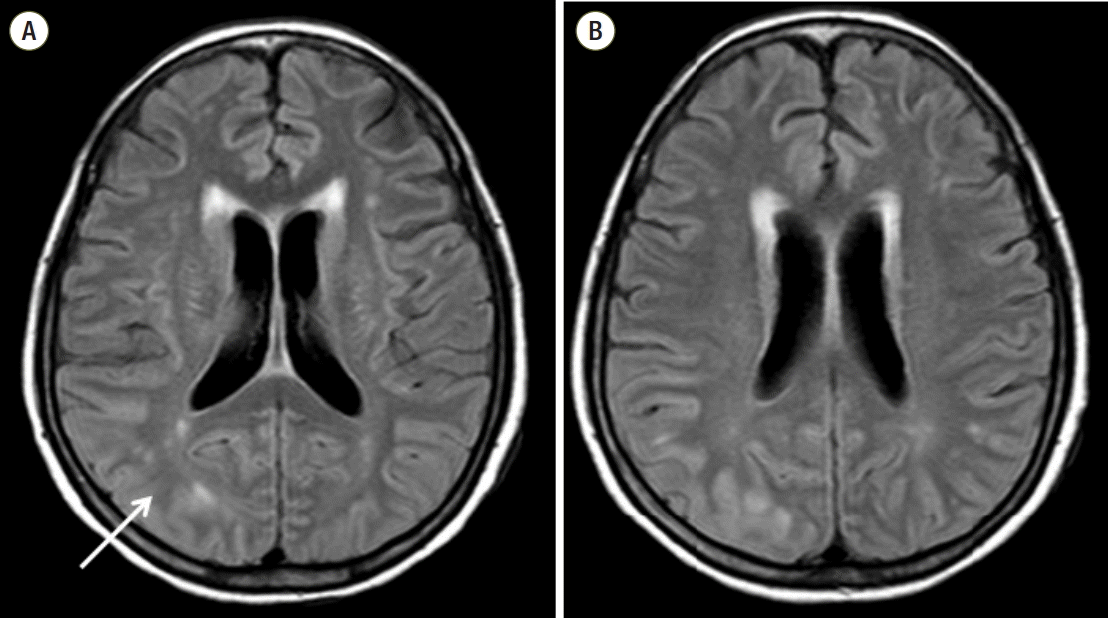
The brain magnetic resonance imaging (MRI) revealed T2 hyperintensity and swelling of the cortex, subcortex of the bilateral temporo-parieto-occipital lobes, dominant in the posterior quadrants (Fig. 3), so neurologists suspected posterior reversible encephalopathy syndrome (PRES). They recommended the dose reduction or substitution of cytotoxic agents. The surgical team reduced the dose of tacrolimus to half dose for 2 days (2 mg per day). They reduced to the quarter dose following 2 days. After symptoms relieved, dose was increased to 1 mg twice a day (Fig. 2).
On POD #19, midazolam infusion was stopped, and her mental status resolved to be alert. So, the extubation was done in the next day. One day later, she was transferred to the general ward.
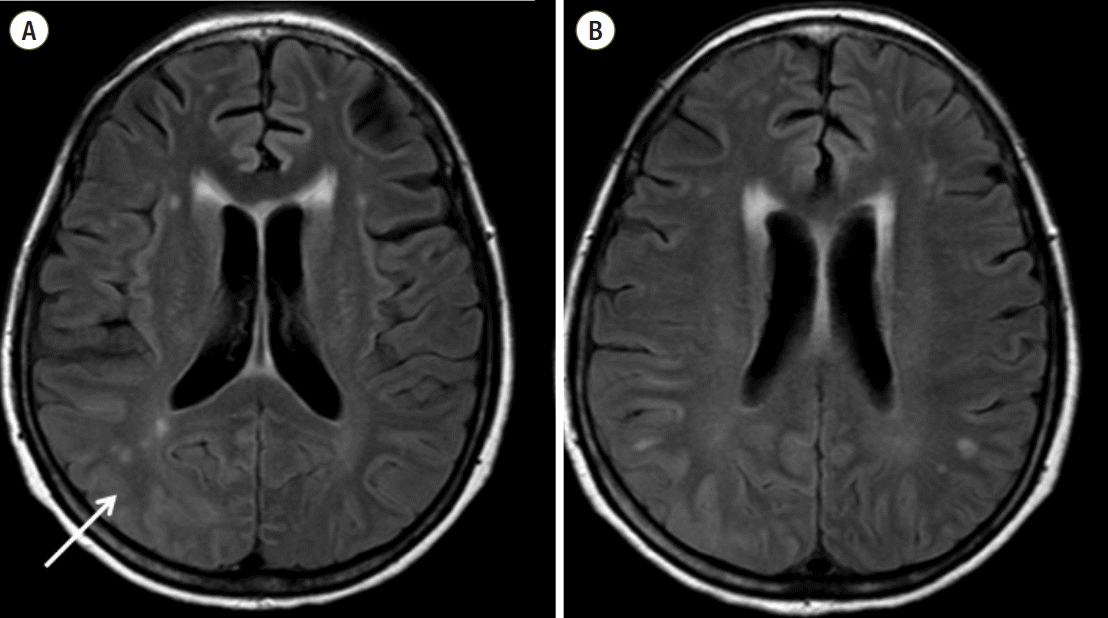
On POD #27, brain MRI showed the improvement of T2 hyperintensity and swelling of the cortex, subcortex of the bilateral temporo-parieto-occipital lobes than before (Fig. 4). She continued to take anti-epileptic drugs at a reduced dose (Fig. 2). On POD #32, EEG revealed the successful treatment of epilepticus. On POD #36, she was discharged without complications.
Go to : 
The neurological complications after LT are more common than other solid organ transplantation, and include seizures, delirium, impaired consciousness, or focal neurological signs about 13-47%.[5] We should be alert on these symptoms, because they are associated with the prolonged hospital stay and decreased post-LT survival.[4,6]
The status epilepticus is widely defined as a prolonged seizure or multiple seizures with incomplete return to baseline. [7] It is a neurological emergency.[8] Especially, patients undergoing LT have high risk for seizures because of neurotoxicity induced by metabolic encephalopathy, immunosuppressant medication, and cerebrovascular complications.[2] Medical team should try to find the causes and correct them rapidly to make patients reversible.
The most important thing is maintaining the stable vital signs, because malignant hypertension or wide blood pressure fluctuation causes adverse effect on central nervous system (CNS).
Patients with new-onset seizure should be measured immediately about serum calcium, glucose, magnesium, phosphorus, sodium, complete blood count, and immunosuppressive medication level. Also, blood and urine cultures should be performed. Brain MRI should be considered to exclude newly developed CNS diseases. CSF test is necessary to exclude CNS infection in especially immune-compromised transplant patients.[9] EEG is required to rule out non-convulsive status epilepticus and diagnosis the mechanism of seizure.
There many possible causes for abrupt seizures after LT as follows: The first cause is metabolic abnormalities. If the function of transplant liver is not resolved, or patients have multi-organ failures, they are prone to the imbalance of electrolytes. Hyponatremia, hypomagnesemia, hypocalcemia, and hypoglycemia should be corrected. But her laboratory results showed mild hyponatremia (129 mmol/L) and hyperglycemia (250 mg/dL) without other abnormalities (ammonia 53 ug/dL, ionized calcium 4.94 mg/dL, ionized magnesium 1.09 mg/dL, aspartate transaminase/alkaline phosphatase 24/42 IU/L).
The second cause is infection. If patient has infection signs like fever, and leukocytosis, the source of infection should be closely inspected. We always have to remember that the transplant patients are very vulnerable to infection. Her laboratory test showed leukocytosis, but she has no source of infection.
The Third, cerebrovascular diseases should be ruled out; cerebral hemorrhage, ischemic stroke, and subdural hematoma.[4] Especially, coagulopathy is frequently associated with liver transplant patients. So, patients who have thrombocytopenia or prolonged clotting time should be monitored closely.[10] In this case, she had no thrombocytopenia (358×103/uL) and normal prothrombin time (International Normalized Ratio 0.92). Additionally, there was no sign of hemorrhage or infarction in the brain image study.
The Fourth, PRES is associated with immunosuppressed state in patients with organ transplantation using cyclosporine and tacrolimus (0.49%).[11] This calcineurin inhibitor (CNI)-induced neurotoxicity presents seizures, altered metal status, headache, and focal neurological deficits including visual loss, or stupor.[9] The risk factors of CNI-induced neurotoxicity are infection, hypomagnesemia, alcoholic liver disease, pretransplant hepatic encephalopathy, posttransplant hyponatremia, and surgical time greater than 7 hours.[10,12] Treatment for PRES is changing of immunosuppressive drugs, and it should be done cautiously in order to minimize the risk of early allograft rejection.[10] Switching to cyclosporine or rapamycin is an effective method for stopping seizures.[13] However, in this case, medical team did not change CNI. Instead, they did reduce the dose of CNI. The concentration of tacrolimus was lower than target level of the early period (10-20 ng/mL). She had hepatic encephalopathy in the preoperative period, received massive transfusion and experienced long operation time. These risk factors could affect her CNS even at normal CNI level and cause status epilepticus.[9,14] Additionally, there are many risk factors of seizures and they are categorized by three parts; genetic factors (Dravet syndrome, glucose transporter 1 deficiency syndrome, Doose syndrome, etc.), structural-metabolic factors (stroke, perinatal insults, neoplasia, traumatic brain injury, infections, malformations of cortical or other brain development, degenerative neurologic diseases, metabolic or toxic insults to the brain, etc.) and unknown factors.[15]
After seizure events, the antiepileptic drugs were given promptly, and the process of diagnosis was performed appropriately, so neurologic state was able to be reversible. To terminate status epilepticus, lorazepam (0.1 mg/kg) is the first-line anti-status agent (typically 1-2 mg in adults, with an additional 2 mg/min, maximum of 8 mg).[9,16] Levetiracetam is the drug of choice for primary generalized seizure in post-transplant patients because it has no hepatic metabolism or drug-drug interactions.[9]
The status epilepticus following LT is not common neurological complications. It is very difficult to discover the primary cause, because the transplant patients have many risk factors and abnormal laboratory findings. However, seizure must be treated promptly to prevent recurrence and further neurological deficits. The medical team has to know the previously mentioned etiology,[9] and be more cautious to manage the seizure after LT.
Go to : 
References
1. Lee SG. A complete treatment of adult living donor liver transplantation: a review of surgical technique and current challenges to expand indication of patients. Am J Transplant. 2015; 15:17–38.

2. Zivković SA. Neurologic complications after liver transplantation. World J Hepatol. 2013; 5:409–16.
3. Bronster DJ, Emre S, Boccagni P, Sheiner PA, Schwartz ME, Miller CM. Central nervous system complications in liver transplant recipients--incidence, timing, and long-term follow-up. Clin Transplant. 2000; 14:1–7.
4. Vizzini G, Asaro M, Miraglia R, Gruttadauria S, Filì D, D’Antoni A, et al. Changing picture of central nervous system complications in liver transplant recipients. Liver Transpl. 2011; 17:1279–85.

5. Senzolo M, Ferronato C, Burra P. Neurologic complications after solid organ transplantation. Transpl Int. 2009; 22:269–78.
6. Lewis MB, Howdle PD. Neurologic complications of liver transplantation in adults. Neurology. 2003; 61:1174–8.

8. Logroscino G, Hesdorffer DC, Cascino G, Hauser WA, Coeytaux A, Galobardes B, et al. Mortality after a first episode of status epilepticus in the United States and Europe. Epilepsia. 2005; 46 Suppl 11:46–8.

9. Shepard PW, St Louis EK. Seizure treatment in transplant patients. Curr Treat Options Neurol. 2012; 14:332–47.

10. Cruz RJ Jr, DiMartini A, Akhavanheidari M, Iacovoni N, Boardman JF, Donaldson J, et al. Posterior reversible encephalopathy syndrome in liver transplant patients: clinical presentation, risk factors and initial management. Am J Transplant. 2012; 12:2228–36.
11. Bartynski WS, Tan HP, Boardman JF, Shapiro R, Marsh JW. Posterior reversible encephalopathy syndrome after solid organ transplantation. AJNR Am J Neuroradiol. 2008; 29:924–30.

12. Balderramo D, Prieto J, Cárdenas A, Navasa M. Hepatic encephalopathy and post-transplant hyponatremia predict early calcineurin inhibitor-induced neurotoxicity after liver transplantation. Transpl Int. 2011; 24:812–9.

13. Sevmis S, Karakayali H, Emiroglu R, Akkoc H, Haberal M. Tacrolimus-related seizure in the early postoperative period after liver transplantation. Transplant Proc. 2007; 39:1211–3.

14. Zhao ZY, He F, Gao PH, Bi JZ. Blood transfusion-related posterior reversible encephalopathy syndrome. J Neurol Sci. 2014; 342:124–6.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download