Abstract
Background
An automatic alarm system was developed was developed for unexpected vital sign instability in admitted patients to reduce staffing needs and costs related to rapid response teams. This was a pilot study of the automatic alarm system, the medical emergency system (MES), and the aim of this study was to determine the effectiveness of the MES before expanding this system to all departments.
Methods
This retrospective, observational study compared the performance of patients admitted to the pulmonary department at a single center using patient data from three 3-month periods (before implementation of the MES, December 2013-February 2014; after implementation of the MES, December 2014-February 2015 and December 2015-February 2016).
Results
A total of 571 patients were admitted to the pulmonary department during the three observation periods. During this pilot study, the MES automatically issued 568 alarms for 415 admitted patients. There was no significant difference in the rate of cardiopulmonary resuscitation (CPR) before and after application of the MES. The mortality rate also did not change. However, it appeared that CPR was prevented in four patients admitted from the general ward to the intensive care unit (ICU) during MES implementation. The median length of hospital stay and median length of ICU stay were not significantly different before and after MES implementation.
Conclusions
Although we did not find a significant improvement in outcomes upon MES implementation, the CPR rate and mortality rate did not increase despite increased comorbidities. This was a small pilot study and, based on these results, we believe that the MES may have significant effects in longer-term and larger-scale studies.
Some patients experience serious adverse events while in the hospital, including sudden cardiac arrest, respiratory failure, acute changes in consciousness, unplanned intensive care unit (ICU) admission, and sudden death [1]; these may lead to irreversible organ damage, increased mortality rate, prolonged hospital stay, and increased medical costs [2,3]. Many studies have reported on the epidemiology and causes of unexpected adverse events in the hospital [2,4-6]. One of the main causes of such adverse events is insufficient, delayed, or incorrect medical detection systems [4,7]. To overcome these problems in medical detection, rapid response systems (RRSs) have been introduced, including the formation of rapid response teams, also known as medical emergency teams or critical care outreach [8,9].
Several studies have shown results, such as decreases in hospital mortality and increases in better care for acutely ill patients, after implementation of an RRS [10-12]. However, RRSs also have shortcomings. The RRS team commonly requires additional staff consisting of critical care staff or fellows, nurses, and respiratory therapists, who can resolve patients’ critical problems [9,13,14]. Maintaining the RRS generates additional expenses including educational expenses and communication system development costs. Furthermore, the RRS may lower the sense of responsibility for patients and interest from the primary doctor [9,15-18].
Our institute has not yet implemented an RRS owing to various limitations, including cost and lack of staff. Instead, we developed an automatic alarm system for unexpected unstable vital signs in admitted patients in the general ward. This automatic alarm system makes the best use of existing resources, such as primary doctors, including residents, nurses, electronic medical record systems (EMRs), and electronic communication systems. We have termed this the “medical emergency system” (MES). The aim of this pilot study was to determine the effectiveness of the MES before expanding this system to all of our departments. The primary objective was to prevent pre-ICU cardiac arrest and decrease mortality rates.
This was planned as a pilot study prior to expanding the MES to all departments in our institute. This was a retrospective, observational study comparing the performance of patients admitted to only one ward on the pulmonary department at a 3,000-bed (30-bed medical intensive care unit) university tertiary referral hospital in Seoul, Korea, with retrospective data from the same hospital before the application of the MES during the winter season. This study was conducted over three 3-month periods (before implementation of the MES, December 2013-February 2014; after implementation of the MES, December 2014-February 2015 and December 2015-February 2016). The primary outcomes were the rate of cardiopulmonary resuscitation (CPR), mortality rate, and ICU admission rate. Length of hospital stay and length of ICU stay were also analyzed.
The MES is an automatic alarm system that detects warning signs of disease progression or adverse events in patients and generates an appropriate awareness for primary care physicians. The MES uses the EMR and existing communication system. Figure 1 shows the design of the MES. In brief, if vital signs entered in the EMR by a patient’s nurse satisfy the criteria for MES, the MES will automatically alert the primary doctor, resident, and on-call doctor of the abnormal vital signs. MES inclusion criteria include abnormal respiration rate, oxygen saturation, heart rate, and systolic blood pressure (Table 1). The MES is not turned on by patients who agree to “do not resuscitate,” are younger than 18 years, or are admitted in the emergency room or ICU. Any doctor who receives the MES message manages the patient according to the MES manuals (Figure 2). After management, the doctor records the method of management, status of the patient, and the results of management. The doctor can then turn off the MES. The MES includes education for primary care physicians that relates to basic procedures and plans for situations such as acute respiratory distress, shock, and arrhythmia. Education was conducted periodically before and after implementing the MES.
The data from patients admitted to one ward of the pulmonary department were collected from the hospital electronic medical records. Clinical data on hospitalization path, length of stay, admission to the ICU, development of CPR, and mortality were evaluated. The Charlson comorbidity index (CCI) was calculated for evaluation of comorbidity [19]. Additionally, we inspected progress of patients admitted to the ICU after implementation of the MES.
Statistical analysis was performed using SPSS version 23.0 (IBM Corp., Armonk, NY, USA). All continuous variables are expressed as mean with standard deviation or median with interquartile range. The chi-square test or Fisher exact test was used to assess differences among the groups. Continuous variables were analyzed using Kruskal-Wallis test or analysis of variance.
A total of 571 patients were admitted to one ward of the pulmonary department during the observation periods. The mean age of the patients was 64.2 years (range, 18-93 years) and 338 of the patients were male (59.2%). One hundred fifty-six patients were admitted in December 2013-February 2014 before implementation of the MES. The 203 patients hospitalized in December 2014-February 2015 and the 212 patients admitted in December 2015-February 2016 were under the MES.
The number of hospitalized patients increased in 2014 and 2015. The sex, mean age, mean body mass index, and hospitalization path (such as emergency department and outpatient) were not significantly different between groups (Table 2). However, the CCI, indicating comorbidity to predict short- and long-term mortality, was significantly different between the groups (P = 0.038), and was significantly higher in 2015 than in 2013 (P = 0.032) (Table 2).
Table 3 shows the results of the analysis before and after implementing the MES. During this pilot study, the MES automatically turned on 568 alarms for 415 admitted patients. Among 568 alarms, 170 (29.9%) were caused by problems in respiration rate; 149 (26.2%) were caused by low oxygen saturation; 77 (13.6%) were caused by problems in heart rate; and 172 (30.3%) were caused by low systolic blood pressure. The response rate to MES alarms increased in the second year of MES implementation compared to the first year (82.7% vs. 43.79%). There was no significant difference in the rate of development of CPR. The mortality rate also did not differ between groups (P = 1.000). The admission rate of the ICU increased, but this was not statistically significant. The majority of patients were admitted to the ICU for ventilator care (2013-2014, 80%; 2014-2015, 80%; 2015-2016, 61%). Among them, four patients admitted from the general ward to the ICU in December 2015-February 2016 underwent CPR with 1 day of ICU admission.
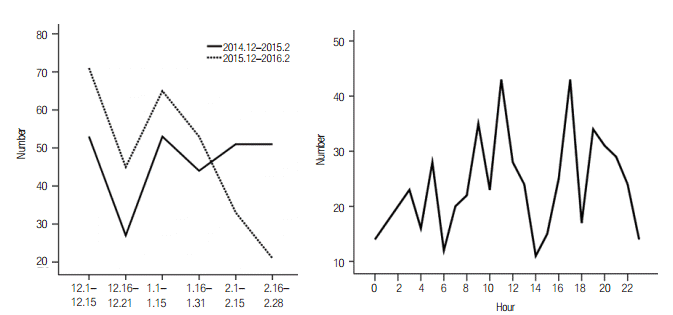
The median length of hospital stay and median length of ICU stay among all of the patients were not significantly different between the groups. However, there were differences in hospitalized days on excluding patients admitted to the ICU and length of ICU stay on ICU-admitted patients. Figure 3 shows the trend of the MES to turn on according to time.
This study examined the effects of the introduction of the MES, an automatic alarm system for unexpected unstable vital signs of patients on the general ward, through the composite incidence of CPR, ICU admissions, and mortality. Our study showed similar CPR rates before and after implementation of the MES. There were no significant differences in mortality of admitted patients or rate of ICU admission. Most studies on RRSs analyzed a large number of subjects and showed low CPR and mortality rates [1,2,9-15]. As the number of subjects in our study was relatively small compared to that in other studies, the results of CPR rate and mortality rates in our study were hard to accept as they are. However, our study showed that the number of admitted patients increased, as did comorbidity of the admitted patients, after implementation of the MES, but the rates of development of CPR and mortality remained the same. Additionally, as the response rate to MES alarms increased, the number of ICU admission also increased. We believe that the MES provides early detection of problems, aiding in the decision to admit patients to the ICU before the development of unexpected adverse events. In agreement with this hypothesis, four patients admitted to the ICU after MES implementation underwent CPR within 1 day of admission.
The strong point of the MES is that it needs only an initial system construction cost and a low maintenance cost and does not require an additional workforce. The weakness of the MES is that necessity of more education can make startup difficult. Without educated physicians, the MES is only a simple alarm clock. To overcome this, our institution paid attention to periodic education for healthcare workers, and the response rate to the MES gradually increased after repeated education. Furthermore, repetitive education imbued healthcare workers with the ability to manage emergencies and it suggests that staff properly trained under the MES could efficiently cope with simultaneous emergencies on multiple wards.
As this was a pilot study, it had several limitations. First, this study was retrospective in design; thus, we could not systematically analyze multiple factors. As this study does not have many variables, we did not know whether multiple variables were correlated. Second, the patients in this study were limited to admission of one ward on the pulmonary department. That ward had relatively low severity compared to other pulmonary departments because it included rooms for patients undergoing bronchoscopy with biopsy. Furthermore, healthcare workers, including the doctors and nurse of the pulmonology ward, are better trained than workers in other department wards, as they see many patients with serious illnesses who need more monitoring. This may have affected the results of this study. Third, the number of subjects in our study was relatively small compared to other studies. Fourth, our observation and implementation time may be too short. Some studies have reported that significant effects of RRSs did not emerge until 2 years after implementation [20,21]. Our study did not find any long-term effects of the MES, because we did not perform continuous studies.
Although we did not find significant improvement in our primary outcomes with MES implementation, the CPR rate and mortality rate did not increase, despite increased comorbidity of patients. However, this study is a pilot study, so we expect that such limitations may be overcome after expanding the MES to all departments in our hospital. Thus, we believe that the MES stands a better chance of significant effects with expansion and increased duration. We will continue to expand the MES to all departments in our hospital.
References
1. Kause J, Smith G, Prytherch D, Parr M, Flabouris A, Hillman K, et al. A comparison of antecedents to cardiac arrests, deaths and emergency intensive care admissions in Australia and New Zealand, and the United Kingdom: the ACADEMIA study. Resuscitation. 2004; 62:275–82.
2. McQuillan P, Pilkington S, Allan A, Taylor B, Short A, Morgan G, et al. Confidential inquiry into quality of care before admission to intensive care. BMJ. 1998; 316:1853–8.

3. McGloin H, Adam SK, Singer M. Unexpected deaths and referrals to intensive care of patients on general wards: are some cases potentially avoidable? J R Coll Physicians Lond. 1999; 33:255–9.
4. Davis P, Lay-Yee R, Briant R, Ali W, Scott A, Schug S. Adverse events in New Zealand public hospitals I: occurrence and impact. N Z Med J. 2002; 115:U271.
5. Thomas EJ, Studdert DM, Burstin HR, Orav EJ, Zeena T, Williams EJ, et al. Incidence and types of adverse events and negligent care in Utah and Colorado. Med Care. 2000; 38:261–71.

6. Brennan TA, Localio AR, Leape LL, Laird NM, Peterson L, Hiatt HH, et al. Identification of adverse events occurring during hospitalization. A crosssectional study of litigation, quality assurance, and medical records at two teaching hospitals. Ann Intern Med. 1990; 112:221–6.
7. Leape LL, Brennan TA, Laird N, Lawthers AG, Localio AR, Barnes BA, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991; 324:377–84.
8. Devita MA, Bellomo R, Hillman K, Kellum J, Rotondi A, Teres D, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Mede. 2006; 34:2463–78.

9. Jones DA, DeVita MA, Bellomo R. Rapid-response teams. N Engl J Med. 2011; 365:139–46.
10. Chan PS, Jain R, Nallmothu BK, Berg RA, Sasson C. Rapid response teams: a systematic review and meta-analysis. Arch Intern Med. 2010; 170:18–26.
11. Hillman K, Chen J, Cretikos M, Bellomo R, Brown D, Doig G, et al. Introduction of the medical emergency team (MET) system: a cluster-randomised controlled trial. Lancet. 2005; 365:2091–7.
12. Priestley G, Watson W, Rashidian A, Mozley C, Russell D, Wilson J, et al. Introducing critical care outreach: a ward-randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004; 30:1398–404.

13. Jones CM, Bleyer AJ, Petree B. Evolution of a rapid response system from voluntary to mandatory activation. Jt Comm J Qual Patient Saf. 2010; 36:266–70.241.

14. Gerdik C, Vallish RO, Miles K, Godwin SA, Wludyka PS, Panni MK. Successful implementation of a family and patient activated rapid response team in an adult level 1 trauma center. Resuscitation. 2010; 81:1676–81.

15. Brunsveld-Reinders AH, Ludikhuize J, Dijkgraaf MG, Arbous MS, de Jonge E; COMET study group. Unexpected versus all-cause mortality as the endpoint for investigating the effects of a rapid response system in hospitalized patients. Crit Care. 2016; 20:168.

16. Maharaj R, Raffaele I, Wendon J. Rapid response systems: a systematic review and meta-analysis. Crit Care. 2015; 19:254.

17. Winters BD, Weaver SJ, Pfoh ER, Yang T, Pham JC, Dy SM. Rapid-response systems as a patient safety strategy: a systematic review. Ann Intern Med. 2013; 158(5 Pt 2):417–25.
18. Sandroni C, D’Arrigo S, Antonelli M. Rapid response systems: are they really effective? Crit Care. 2015; 19:104.

19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40:373–83.

Figure 1.
Design of the medical emergency system (MES). When a nurse enters vital sign data into the electronic medical record system, the computer automatically analyzes this information. If vital signs meet the MES criteria, a message is automatically sent to the primary doctor, resident, and on-call doctor. A doctor who receives the message must treat the patient and chart the treatment to deactivate the MES. If the MES is not deactivated, the system will continue to send the message to the doctors. EMR: electronic medical record; SMS: short message service.
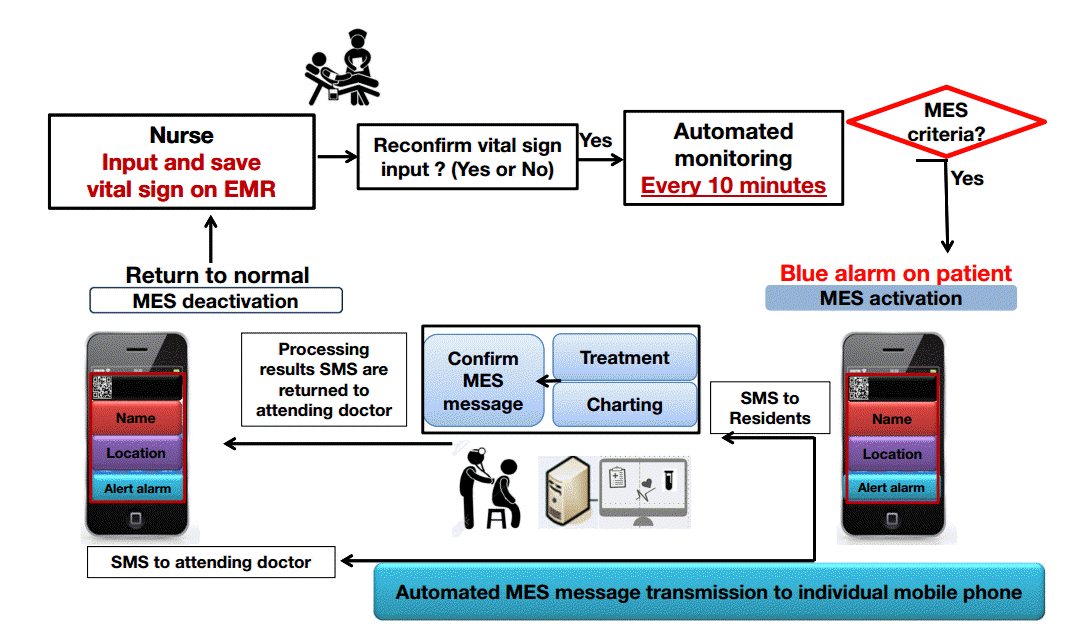
Figure 2.
Protocol for the medical emergency system. Management flow for (A) shock, (B) hypoxemia and tachypnea, (C) tachycardia. SBP: systolic blood pressure; SpO2: oxygen saturation; RR: respiratory rate; HR: heart rate; EMR; electronic medical record; GCS: glasgow coma scale; MES: medical emergency system; qSOFA: quick sepsis related organ failure assessment; CBC: complete blood count; diff: differential count; T.bil: total bilirubin; Cr: creatinine; ABGA: arterial blood gas analysis; EKG: electrocardiogram; CXR: chest X-ray; MAP: mean arterial pressure; ICU: intensive care unit; Tx: treatment; HAT; hypotension, altered mental status, tachypnea; BP: blood pressure; Resp: respiration; COPD: chronic obstructive pulmonary disease; PTE: pulmonary thromboembolism; CT: computed tomography; TTE: transthoracic echocardiogram; AVNRT: atrioventricular nodal reentrant tachycardia; AVRT: atrioventricular reentrant tachycardia; VT: ventricular tachycardia; VF: ventricular fibrillation; DDx: differential diagnosis; A-fib: atrial fibrillation; PSVT: paroxysmal supraventricular tachycardia. aEye response, 1–4; verbal response,1–5; motor response, 1–6.
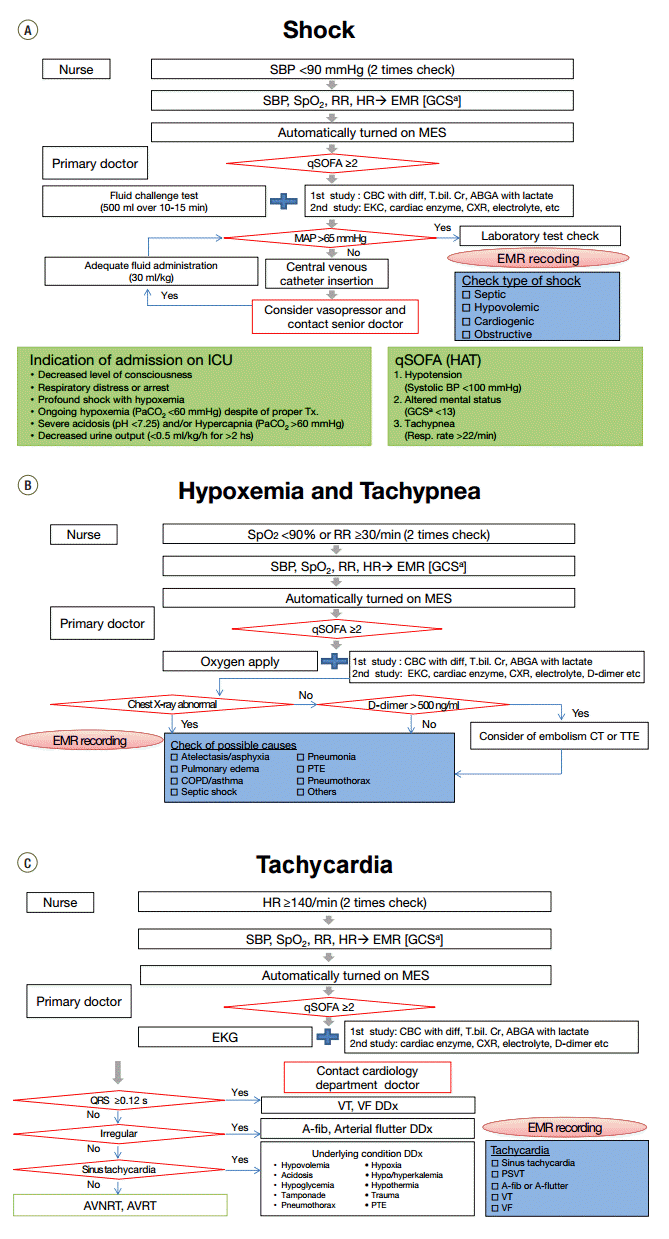
Table 1.
Medical emergency system inclusion criteria
Table 2.
Baseline characteristics of total patients
Table 3.
Outcomes after implementation of medical emergency system




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download