Abstract
ACKNOWLEDGMENTS
References
Fig. 1.
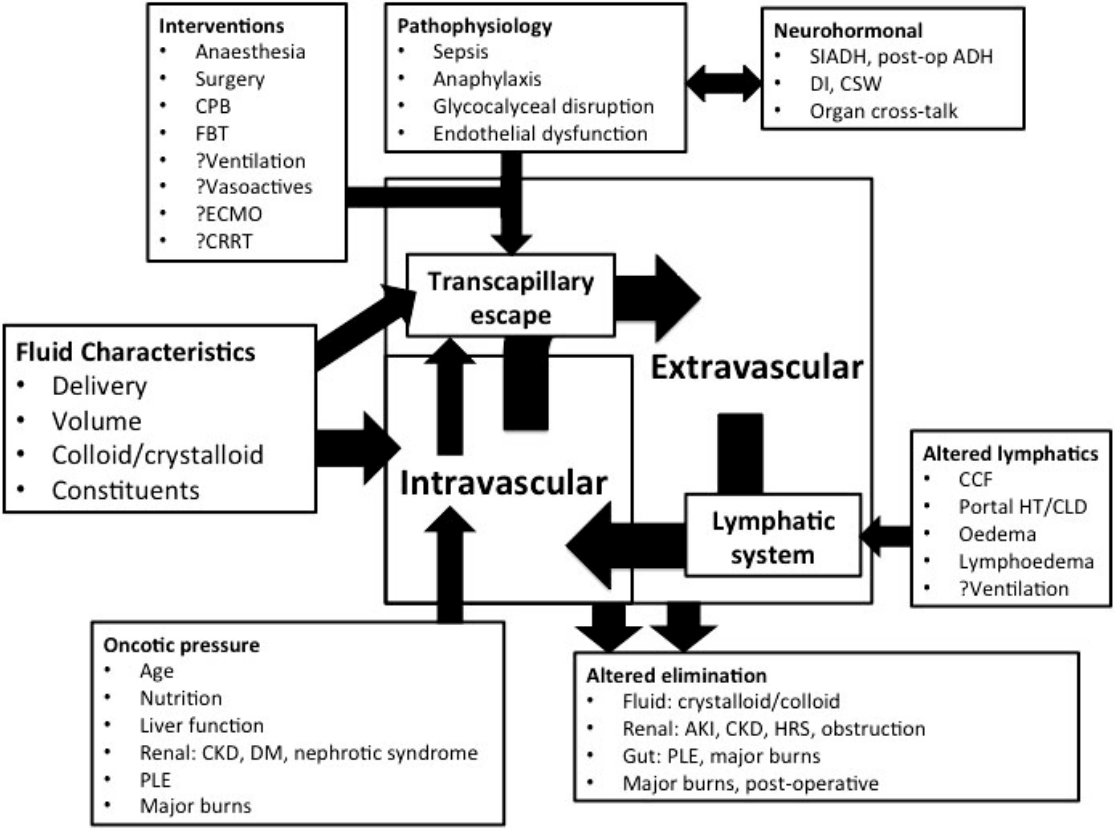
Fig. 2.
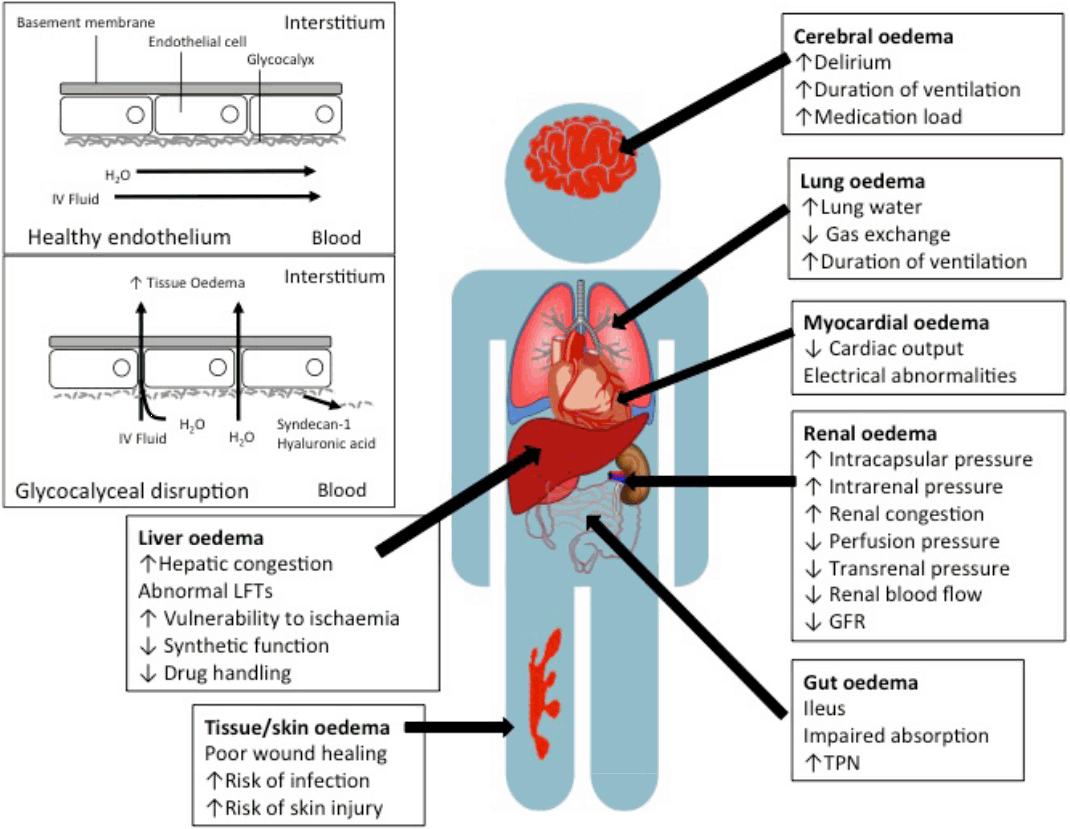
Table 1.
|
Details |
0-6 h interventions |
6-72 h interventions |
Mortality |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Group | n | APACHE II | Total IVF* (mL) | MV† (%) | RBC‡ (%) | VP§ (%) | DOB∥ (%) | Total IVF* (mL) | MV† (%) | RBC‡ (%) | VP§ (%) | DOB∥ (%) | 28 d¶ (%) | 60 d** (%) | 90 d† (%) |
| Rivers [42] | EGDT | 130 | 21.4 ± 6.9 | 4,981 ± 2,984 | 53 | 64.1 | 27.4 | 13.7 | 8,625 ± 5,162 | 55.6 | 74.2 | 56.5 | 28.2 | 33.3 | 44.3 | |
| Usual | 133 | 20.4 ± 7.4 | 3,499 ± 2,438 | 53.8 | 18.5 | 30.3 | 0.8 | 10,602 ± 6,216 | 70.6 | 41.3 | 73.2 | 9.2 | 49.2 | 56.9 | ||
| ProCESS [54] | EGDT | 439 | 20.8 ± 8.1 | 2,805 ± 1,957 | 26.4 | 14.4 | 54.9 | 8 | 4,458 ± 3,878 | 33.7 | 19.8 | 47.6 | 4.3 | 21 | 31.9 | |
| Standard | 446 | 20.6 ± 7.4 | 3,285 ± 1,743 | 24.7 | 8.3 | 52.2 | 1.1 | 4,918 ± 4,308 | 31.4 | 20.9 | 46.6 | 2 | 18.2 | 30.8 | ||
| Usual | 456 | 20.7 ± 7.5 | 2,279 ± 1,881 | 21.7 | 7.5 | 44.1 | 0.9 | 4,354 ± 3,882 | 27.9 | 18 | 43.2 | 2.2 | 18.9 | 33.7 | ||
| ProMISe [53] | EGDT | 356 | 20 ± 6.9 | 2,226 ± 1,443 | 20.2 | 8.8 | 53.3 | 18.1 | 4,215 ± 3,068 | 24.4 | 12.6 | 57.9 | 17.7 | 24.8 | 29.5 | |
| Usual | 332 | 19 ± 7.1 | 2,022 ± 1,271 | 19 | 3.8 | 46.6 | 3.8 | 4,366 ± 3,114 | 25.4 | 8.5 | 52.6 | 6.5 | 24.5 | 29.2 | ||
| ARISE [52] | EGDT | 792 | 15.4 ± 6.5 | 1,964 ± 1,415 | 22.2 | 13.6 | 66.6 | 15.4 | 4,274 ± 3,071 | 27 | 11 | 58.8 | 9.5 | 14.8 | 18.6 | |
| Usual | 796 | 15.8 ± 6.5 | 1,713 ± 1,401 | 22.4 | 7 | 57.8 | 2.6 | 4,382 ± 3,136 | 27 | 11.8 | 51.5 | 5 | 15.9 | 18.8 | ||
EGDT: early goal direct therapy group; ProCESS: Protocolized Care for Early Septic Shock; ProMISe: Protocolised Management of Sepsis; ARISE: Australasian Resuscitation in Sepsis Evaluation; APACHE II: Acute Physiology and Chronic Health Evaluation Score II; IVF: intravenous fluids; MV: mechanical ventilation; RBC: red blood cell transfusion; VP: vasopressor medication; DOB: dobutamine infusion; Usual: usual care group; Standard: protocolised standard care.
Table 2.
| Year | Design and setting | Population | Aim | Associations between fluid and outcomes |
|---|---|---|---|---|
| Adult critical care | ||||
| Adult cardiac surgery | ||||
| Moore, et al. [72] 2015 | Retrospective single-centre observational study in an Australian academic ICU. | 2,171 adult cardiac surgical patients, mean age 67 ± 11 years, 74% male, 81 ± 16 kg in weight, with mean APACHE score 15 ± 4. Excludes those without baseline weight or Cr value. | To assess if correcting Cr measurements for FB would identify more patients with AKI criteria, and the associations between AKI in these patients and clinical outcomes. | Mean FB + 4 L, median + 3.9 L at 18 h post-ICU admission. |
| Additional 11.9% of patients reclassified as AKI + once Cr corrected for FB. | ||||
| Sequentially more patients reclassified with increasing FB quartile. | ||||
| Risk of mortality, length of stay, need for RRT in reclassified patients increased to between baseline and that of the original AKI group. | ||||
| Adult surgical | ||||
| Barmparas, et al. [73] 2014 | Prospective single-centre observational study in a US academic ICU. | 144 adult surgical patients, mean age 55.3 ± 24.8 years, 67.4% male, with APACHE IV score of 37.2 ± 29.2 and 13.2% hospital mortality. | To evaluate the effect of achieving a negative FB on outcomes in a critically ill surgical population. | Negative FB status by D5 ICU independently associated with survival, and by D1 with reduced complications, when adjusted for trauma, illness severity, CVD, DM, VA use, age, CLD, MV, in over-adjusted models given the rate of outcomes. |
| Negative FB D5 ICU or discharge: OR (mortality): 0.31 (95% CI: 0.13-0.76). | ||||
| Elofson, et al. [74] 2015 | Retrospective single-centre observational study in a US academic ICU and trauma centre. | 197 adult mechanically ventilated surgical patients recruited within 24 h of ICU admission and surgical intervention; 101 patients with low FB, 96 with high FB. Low FB patients were younger, fewer co-morbidities, lower illness severity, different surgical presentation. | To determine the impact of postoperative D7 FB on clinical outcomes in critically ill surgical patients following adequate initial fluid resuscitation. | High FB defined as ≥ 5 L + FB by ICU D7; low ≤ 5 L. |
| High FB status by D7 ICU crudely associated with duration of MV, and independently associated with mortality, and by D1 with reduced complications, when adjusted for age, sex, comorbidity, abdominal surgery, illness severity. | ||||
| Significantly more FBT, BP given to high FB patients, with similar VA use. | ||||
| Survivors have significantly lower daily FB from D3 and cumulative FB from D4. | ||||
| High FB: OR (mortality): 12.7 (95% CI: 3.5-45.8). | ||||
| Shim, et al. [75] 2014 | Retrospective single-centre observational study in a Korean academic surgical ICU. | 148 adult surgical patients with admissions ≥ 48 h, median age 67 (20-88) years, 66.9% male, 18% with APACHE II score ≥ 20 and 20.9% hospital mortality. Excluded trauma, transplantation patients, and those requiring repeat operation. | To determine the relationship between fluid balance and mortality in a critically ill surgical population. | In patients with APACHE II scores > 20, survivors demonstrated significantly lower daily and cumulative FB than non-survivors for D1-3 of ICU. |
| Unclear statistical approach, no adjustment for multiple potential confounders, no multivariable modelling, small sample size. | ||||
| Silva, et al. [76] 2013 | Multi-centre (4) prospective observational study from Sao Paulo, Brazil. | 479 adult surgical patients, median age 64 (51-74) years, 51% male, with SAPS 3 score of 41.8 ± 14.5 and 8.8% hospital mortality. Excluded moribund or palliative patients, patients with renal failure, severe heart failure or DM. | To evaluate the impact of intraoperative FB on rates of infection, organ dysfunction, and mortality in the post-operative period. | Non-survivors require more crystalloid and colloid (NS) and have a significantly higher intraoperative FB (+1950 [+1400-+3400] mL compared to +1400 [+1000-+1600] mL in survivors); also report higher rates of intraoperative BP and VA use. |
| Independent association between intraoperative fluid balance and hospital mortality accounting for illness severity, ASA and location. | ||||
| Per 100 mL + FB: OR (mortality): 1.03 (95% CI: 1.01-1.04). | ||||
| Adult Undifferentiated Critical Care | ||||
| Garzotto, et al. [77] 2016 | Multi-centre (21) prospective observational study from 9 European countries. | 1,734 adult ICU patients, 42.8% with AKI, 23.4% with sepsis, severe sepsis or septic shock on admission; mean age 59.2 ± 15.2 years, 65.3% male, mean APACHE II score of 17.1 ± 7.7. Excluded postoperative patients, patients on ECMO or with ESRF, or moribund patients. | To explore the effect of changes in fluid balance throughout ICU stay and RRT on ICU mortality. | Peak FO% was associated independently associated with mortality after adjusting for illness severity and presence of AKI. Per peak FO%: OR (mortality): 1.04 (95% CI: 1.02-1.07). The velocity of fluid accumulation was independently associated with mortality, when adjusted for illness severity, DM, CVD and HT, in patients with AKI or requiring CRRT, but not those without AKI. |
| Patients with AKI: HR (mortality): 1.31 (95% CI: 1.14-1.5)/unit of velocity of fluid accumulation. | ||||
| Patients on RRT: HR (mortality): 1.21 (95% CI: 1.04-1.42)/unit of velocity of fluid accumulation. | ||||
| Shum, et al. [78] 2011 | Retrospective single-centre observational study in an academic ICU in Hong Kong. | 639 adult patients, undifferentiated critically ill cohort with ICU stay > 72 h, 93.9% emergency admissions, mean age 65.5 ± 15.8 years, 59.6% male, with a mean APACHE IV score of 79.8 ± 34.4 and 12.8% hospital mortality. Excluded readmissions, those without illness severity data, those < 16 years. No cardiac surgery, transplantation or burns. | To explore the relationship between cumulative FB during ICU stay, and the outcomes in an undifferentiated critically ill population. | Mean ICU D1 FB 0.73 ± 1.13 L; D2 0.49 ± 1.17 L; D3: 0.18 ± 0.82 L. |
| Daily FB significantly lower on D1-5 in ICU survivors. | ||||
| A positive ICU D1 FB was independently inversely associated with hospital mortality and ICU D2+3 FB and ICU ADM FB were positively independently associated with mortality, when adjusted for age, illness severity, diagnosis, specialty, length of stay, and fluid volume variables. | ||||
| ICU D1 FB: OR (mortality): 0.71 (95% CI: 0.56-0.90). | ||||
| ICU D2+3 FB: OR (mortality): 1.54 (95% CI: 1.22-1.96). | ||||
| ICU ADM FB: OR (mortality): 1.31 (95% CI: 1.17-1.48). | ||||
| ICU ADM FB AUROC (mortality): 0.81 (95% CI: 0.76-0.84), better than APACHE. | ||||
| Adult Sepsis | ||||
| Boyd, et al. [49] 2011 | Post-hoc analysis of multicentre (27) RCT data from 3 countries (from the Vasopressin versus Norepinephrine Infusion in Patients with Septic Shock trial). | 778 severely unwell adult patients with septic shock, age ≈ 60 years, 60% male, with an APACHE II score ≈ 27 and 37.2% 28 d mortality Excluded delayed recruitment > 24 h, malignancy or terminal disease, ischaemic gut, acute or chronic heart disease, hyponatraemia, TBI, pregnancy, vasospastic conditions, those < 16 years. | To explore the relationship between CVP and FB and mortality following resuscitation for septic shock. | 12 h following enrolment, mean +FB was 4.2 ± 3.8 L, mean IVF given 6.3 ± 3.5 L, mean UO 2.0 ± 2.3 L. |
| Mean daily FB on ICU D1: 1.5 ± 1.8 L; D2: 2.5 ± 2.8 L; D3: 1.4 ± 2.3 L; D4: 0.69 ± 2.1 L. | ||||
| Wettest quartile: median IVF at 12 h: 10,100 (8,430-12,100) mL; on ICU D4 30,600 (26,200-36,000) mL; median FB at 12 h 8,150 (7,110-10,100); on ICU D4 mL 20,500 (17,700-24,500) mL. | ||||
| Driest quartile: median IVF at 12 h: 2,900 (2,050-3,900) mL; on ICU D4 710 (132-1,480) mL; median FB at 12 h 710 (132-1,480) mL; on ICU D4 1,560 (723-3,210) mL. | ||||
| Independent reduction in mortality for those in quartile 1/2 compared to quartile 4 for median 12 h and D4 FB on extensive modelling. | ||||
| ICU 12 h FB: HR (mortality): 0.71 (95% CI: 0.56-0.90) 1st vs. 4th quartile. | ||||
| ICU D4 FB: HR (mortality): 1.54 (95% CI: 1.22-1.96) 1st vs. 4th quartile. | ||||
| Brotfain, et al. [79] 2016 | Retrospective single-centre observational study in an academic ICU in Israel. | 297 adult patients with sepsis, age ≈ 26 to 95 years, ≈ 50% male, with an APACHE II score ≈ 15 to 37 and 40.1% hospital mortality. Excluded patients discharged to other acute or chronic hospitals. | To explore the association of FB and clinical outcomes in septic patients. | 70% patients leaving ICU/dying > 10 L FB+. |
| Mean 72 h FB: G1: 6.67 ± 4.72 L; G2: 10.84 ± 5.12 L; G3: 12.47 ± 5.73 L; G4: 13.76 ± 7.38 L. | ||||
| Mean FB at discharge: G1: 4.89 ± 2.99 L; G2: 15.42 ± 2.65 L; G3: 24.54 ± 2.76 L; G4: 44.4 ± 4.78 L. | ||||
| FB at discharge independently associated with mortality when adjusted for illness severity, tracheostomy, organ dysfunction, and antibiotic use and 72 h FB. | ||||
| Mean FB at discharge: OR (mortality): 1.54 (95% CI: 1.22-1.96) 1st vs. 4th quartile. | ||||
| Cronhjort, et al. [80] 2016 | Secondary analysis of multi-centre (32) RCT data from Scandinavian ICUs. | 841 adult patients with septic shock with ICU admission longer than 72 h, ages ≈ 35-91 years, 54.2% male, with SOFA scores of 3.2-17 and 52% 90 d mortality. Excluded losses to follow-up and missing FB data. | To explore the association between cumulative fluid balance at 72 h and 90 d mortality following participation in a transfusion trial in septic shock. | Median cumulative FB at 72 h was 2480 (47-5,045) mL. |
| Regression analysis based on FB quartiles of < 0/0-29.99/30-74.99/≥ 75 mL/kg. | ||||
| Average cumulative FB low and mortality rate high in comparison to other studies. | ||||
| Kelm, et al. [81] 2015 | Retrospective single-centre observational study in a US academic ICU. | 405 adult patients, 59.8% with septic shock, average age ≈ 70 years, 57.3% male, with an APACHE III score ≈ 59 and 22.9% hospital mortality. Excluded readmissions. | To determine the potential morbidity and mortality associated with FO in patients receiving EGDT. | 67% of patients undergoing EGDT develop clinical evidence of FO on ICU D1, and this persists in 48% of patients. |
| No significant statistical difference in FB between groups, absolute difference of ≈ 900 mL less in FO free group; they also have lower rates of CKD, CHF, CLD and IHD. | ||||
| Evidence of persistent FO at D3 is independently associated with hospital mortality when adjusted for weight and measures of illness severity; also associated with interventions for remove fluid. | ||||
| FO at D3 OR (mortality): 1.92 (95% CI: 1.16-3.22). | ||||
| Adult Acute Lung Injury | ||||
| Azevedo, et al. [82] 2013 | Multi-centre (45) prospective observational study from Brazilian ICUs. | 773 adult patients, 59.8% with septic shock, age ≈ 70 years, 57.3% male, with an APACHE III score ≈ 59 and 42% hospital mortality. Excluded readmissions, those requiring < 24 h MV, moribund or palliative patients, patients with tracheostomies or those who are pregnant. | To describe the clinical outcomes of patients in Brazilian ICUs requiring ventilatory support and identify variables associated with mortality. | ICU D3 median cumulative FB 2.9 (0.8 to 5.4) L. |
| Independent association between cumulative ICU D3 FB and mortality as quartiles when adjusted for age, illness severity, comorbidity, severity of ARDS, NIV failure, need for MV, and lactate. ICU D3 FB > 2 L also independently associated with NIV failure when adjusted for illness severity and ARDS. | ||||
| > 1.5 L – FB at D3 OR (mortality): 3.08 (95% CI: 1.47-6.48). | ||||
| –1.5 to +1.5 L FB at D3 OR (mortality): reference. | ||||
| +1.5 to +5 L FB at D3 OR (mortality): 1.84 (95% CI: 1.11-3.04). | ||||
| > +5 L FB at D3 OR (mortality): 2.44 (95% CI: 1.39-4.28). | ||||
| Adult patients with malignancy | ||||
| de Almeida, et al. [83] 2014 | Retrospective single-centre observational study in an academic oncology ICU in Brazil. | 122 adult patients with cancer admitted to ICU, median age 63 (61-65) years, 55.7% male, median APACHE II score 14 (10-20) and 20.5% hospital mortality. Excluded trauma, transplantation. | To explore the relationship between FB and clinical outcomes in patients with cancer. | Median daily FB for 72 h: 887 (104-1,557) mL/24 h survivors vs. 1,675 (471-2,921) mL/24 h non-survivors. |
| Median daily FB for 72 h > 1100 mL/24 h independently associated with mortality when adjusted for illness severity and lung injury score. | ||||
| Excluded moribund or palliative patients, those with admissions < 24 h, those declining consent, those with ESRF, or with active bleeding, or those enrolled in other studies. | Median daily FB for 72 h > 1100 mL/24 h: OR (mortality): 5.14 (95% CI: 1.45-18.24). | |||
| These patients likely have/develop AKI to accumulate > 1 L/24 h, only partial adjustment in the form of illness severity, not specifically for AKI. | ||||
| Adult AKI | ||||
| Bellomo, et al. [84] 2012 | Post-hoc analysis of multi-centre (35) RCT data from 2 countries (from the Normal vs. Augmented Level of Replacement [RENAL] Therapy trial). | 1,453 critically ill adult patients with AKI requiring RRT, 49.5% with severe sepsis, age ≈ 35 to 90 years, ≈ 64% male, with an APACHE III score ≈ 50 to 158 and 44.3% hospital mortality. Excluded readmissions. | To explore the relationship between FB and primary and secondary predefined study outcomes from the RENAL trial. | Patients with a positive mean daily FB had higher illness severity scores, were more likely to have been admitted as emergencies, and had higher 90 d mortality rates. |
| Mean daily FB: non-survivors: 305 (–274 to 1,116) mL; survivors: –226 (–738 to 255) mL. | ||||
| Mean cumulative FB: non-survivors: 1,518 (–2,310 to 5,922) mL; survivors: –1,928 (–6,863 to 2,240) mL. | ||||
| Independent association between negative mean daily FB in ICU and 90 d mortality, persisting after extensive adjustment and sensitivity analyses, including propensity score analysis. | ||||
| Negative mean daily FB in ICU: OR (mortality): 0.32 (95% CI: 0.24-0.43). | ||||
| Bouchard, et al. [85] 2015 | Multi-centre (14) prospective observational study from ICUs in developed and emerging caintries. | 745 adult patients, 35% surgical, 44.3% with sepsis, 43% white, mean age 60.5 ± 18.4 years, 62.2% male, with a mean APACHE III score of 55.5 ± 26.7 and 22% hospital mortality. Excluded those missing FB data. | To explore and compare the aetiology and epidemiology of AKI in critically ill patients from developed and emerging countries. | Cumulative FB until D7 was 1.44 (–0.91-4.18) L with no difference between developed and emerging countries.Cumulative FB only independently associated with mortality in developed countries - VA use appears to replace in emerging countries; extensive adjustment. FB until D7 OR (mortality): 1.12 (95% CI: 1.05-1.20), developed countries only. |
| de Olivieira, et al. [86] 2015 | Post-hoc analysis of a prospective single-centre observational study in a Brazilian academic ICU. | 116 septic adult patients, 73.2% with septic shock, median age 60 (44-74) years, 63.5% male, with a median APACHE II score of 17 (13-26) and 62.1% hospital mortality. Excluded those missing FB data. | To explore and compare the aetiology and epidemiology of AKI in critically ill patients from developed and emerging countries. | Reports greater FB and lower UO in non-survivors at 24 h and until 48 h; values not given. |
| +FB > 3.4 L independently associated with mortality when adjusted for age and illness severity, but no accounting made for collinearity/interaction of UO. | ||||
| FB ≥ +3.4 L at 24-48 h OR (mortality): 3.19 (95% CI: 1.19-8.54). | ||||
| Grams, et al. [87] 2011 | Post-hoc analysis of multi-centre (20) RCT data from US (from the Fluids and Catheters Treatment [FACTT] trial). | 306 critically ill adult ventilated patients developing AKI within 48 h of randomisation to PAC/CVC and conservative/liberal fluid therapy; mean age ≈ 50 years, ≈ 57% male, with mean APACHE III scores > 100 and ≈ 38% 60 d mortality. Conservative fluid therapy patients (n= 137) had higher FB, lower albumin concentrations, and were less likely to have aspirated. Excluded readmissions. | To explore the relationships between FB, diuretic therapy, AKI and mortality in patients with ALI. | Mean FB 24 hours pre-enrolment significantly different: conservative: 3.2 L vs. liberal: 4.1 L. |
| Mean cumulative FB study: conservative: 3.7 L vs. liberal 10.2 L. | ||||
| Significantly greater furosemide exposure in conservative group. | ||||
| Following the development of AKI, FB was independently associated with an increased risk of mortality, through multiple sensitivity analyses and across gender, fluid strategy, early and late oliguria and severity of AKI groups. | ||||
| Protective effect of furosemide, once adjusted for FB, is no longer significant. | ||||
| Mean FB+/24 h post-AKI: OR (mortality): 1.61 (95% CI: 1.32-1.96). | ||||
| Neyra, et al. [88] 2016 | Retrospective single-centre observational study in a US academic ICU | 2,632 adult ICU patients with severe sepsis or septic shock and appropriate creatinine measurements; 679 (25.8%) patients with AKI + CKD, 846 (32.1%) patients with AKI alone; 22.9% mortality. Excluded patients with stage 5 CKD or on CHD. | To explore the relationship between cumulative FB and hospital mortality in critically ill septic patients, and assess if it was modified by the presence of AKI or CKD. | Cumulative FB at 72 h: AKI + CKD: 4.16 ± 7.34 L; CKD: 2.85 ± 5.91 L; AKI: 5.55 ± 7.50 L; no KD: 2.74 ± 5.31 L. |
| FO% at 72 h: AKI + CKD: 5.7 ± 9.7%; CKD: 4.7 ± 9.1%; AKI: 8.0 ± 11.1%; no KD: 4.0 ± 7.7%. | ||||
| Independent association between cumulative FB at 72 h and mortality after extensive appropriate adjustment. | ||||
| Patients with AKI + CKD: OR (mortality): 1.06 (95% CI: 1.03-1.09)/1000 mL FB+ at 72 h. | ||||
| Patients with CKD: OR (mortality): 1.09 (95% CI: 1.05-1.13)/1000 mL FB+ at 72 h. | ||||
| Patients with AKI: OR (mortality): 1.05 (95% CI: 1.03-1.08)/1000 mL FB+ at 72 h. | ||||
| Patients with no KD: OR (mortality): 1.07 (95% CI: 1.02-1.08)/1000 mL FB+ at 72 h. | ||||
| Teixeira, et al. [89] 2013 | Secondary analysis of multi-centre (10) prospective observational study from Italian ICUs. | 132 critically ill patients with AKI, 11.4% with sepsis on admission, median age 68.5 (38-75.5) year, 63.6% male, median APACHE II score of 21 (16-28). | To explore the impact of FB and UO on outcome and if they act as independent predictors of mortality in the setting of AKI. | Median FB: non-survivors: 1.09 (0.49 to 1.85) L; survivors: 0.02 (-0.17 to 0.48) L. |
| Excluded patients with incomplete FB data, and those with ESRF. | Both mean FB and mean UV, when adjusted for sepsis, age, gender, illness severity, DM, CVD and HT, and interaction term of mean FB and mean UV, were independently associated with mortality. | |||
| Patients with AKI: HR (mortality): 1.33 (95% CI: 1.002-1.77) per litre/day of mean FB. | ||||
| Vaara, et al. [90] 2012 | Multi-centre (17) prospective observational study from 17 Finnish ICUs. | 296 critically ill adult patients with AKI requiring RRT, 48% with severe sepsis, median age 64 (55-73), 66.6% male, with a median SAPS II score of 51 (40-65) and 39.2% 90 d mortality. Excluded readmissions, previous RRT or ESRF, those with admissions < 24 h, those declining consent, those with incomplete FB data. | To explore the association of factors around RRT initiation with cumulative FB and 90 d mortality in critically ill adults. | 27% of patients develop FO > 10% prior to CCRT, greater fluid accumulation, FB, illness severity. |
| Cumulative FB at RRT initiation: survivors: 3.1 (0.3-6.4) L; non-survivors: 6.2 (2.2-9.7) L. | ||||
| FO% at RRT initiation: survivors: 3.6 (0.3-8.2) %; non-survivors: 8.0 (3.0-12.9) %. | ||||
| Significant, dose-response type effect of pre-RRT FO% and mortality. | ||||
| FO > 10% independently associated with mortality after robust and extensive adjustment. | ||||
| FO > 10% at RRT initiation: OR (mortality): 2.63 (95% CI: 1.3-5.3). | ||||
| Paediatrics | ||||
| Paediatric cardiac surgery | ||||
| Seguin, et al. [91] 2014 | Retrospective single-centre observational study in a Canadian academic paediatric ICU. | 176 paediatric patients (193 surgeries) following cardiac surgery, mean age 2.6 ± 4.2 years, 62% male, with Aristole scores ≈ 4-13, and 2.2% hospital mortality. Excluded neonates undergoing surgery for PDA. | To describe the epidemiology of FO after cardiac surgery and if it is associated with worse clinical and respiratory outcomes in this population. | Median of 30% of all IVF administered as FBT over ICU D1-2, 50% BP. |
| Peak cumulative FO% 7.4 ± 11.2% on ICU D2, independently associated with early IVF administration, cyanotic heart disease and first operation. | ||||
| D2 FO% was independently associated with greater PICU length of stay and duration of MV, adjusting for multiple clinical variables. | ||||
| Paediatric critical care | ||||
| Ketheranathan, et al. [92] 2014 | Retrospective single-centre observational study in a South African academic paediatric ICU. | 100 paediatric patients, median age 9 (2-39) months, 60% male, with PIM II score of 7 (3-20) and 10% hospital mortality. | To assess the prevalence of FO and mortality, and the relationship between FO and patient centred outcomes and ventilatory parameters in this population. | Greater volumes of PICU FBT and IVF given to non-survivors (12.5 vs. 0 mL/kg and 120-150 vs. 60-95 mL/kg/day). |
| Univariate associations demonstrated between FO and 28 day mortality with oxygenation indices, VA use, duration of ventilation, GFR and PICU LOS. Meaningful multivariable regression precluded by sample size. | ||||
| Li, et al. [93] 2016 | Prospective single-centre observational study in a Chinese academic paediatric ICU, | 370 paediatric patients staying in the PICU for ≥ 24 h, median age 11 (3-33) months, 64.3% male, with PRISM III score of 3 (0-6) and 4.9% hospital mortality. Excluded premature neonates or children > 16 years, or those with incomplete FB data. | To explore risk factors for early FO on ICU D1 and its’ association with AKI and mortality in the undifferentiated PICU population. | 17.3% developed early FO defined as fluid accumulation ≥ 5% by 24 h. |
| On Cox regression, independent association between mortality and FO > 5% on D1 ICU (HR 7.27), when adjusted for illness severity, age, MV and AKI. | ||||
| Attempts to use FO and AKI alone and in combination to predict mortality and compare AUROCs to PRISM III - collinear as score contains UO and Cr components. | ||||
| D1 ICU FO%: OR (mortality): 1.17 (95% CI: 1.01-1.37) per %. | ||||
| Paediatric Sepsis/Septic Shock | ||||
| Abulebda, et al. [94] 2014 | Multi-centre (17) retrospective observational study from US academic paediatric ICUs. | 317 paediatric patients with septic shock, < 10 years of age, 57% male, all requiring respiratory support, with PRISM III scores ≈ 7 to 37 and 12.6% hospital mortality. | To explore the relationship between post-PICU admission FB and outcome following admission with septic shock. | Median IVF given and %FB+ at 24 h, and cumulative %FB+ greater in nonsurvivors. |
| Univariate associations demonstrated between cumulative %FB+ and mortality only among patients at low risk of mortality. | ||||
| Meaningful multivariable regression precluded by collinearity. | ||||
| Bhaskhar, et al. [95] 2015 | Retrospective single-centre observational study in a US academic paediatric ICU. | 114 paediatric patients staying in the PICU for ≥ 48 h, median age 1.1 (0-17.4) years, 67% male, with PIM II score of 5.1 (0.2-99.3) and 13% hospital mortality. Excluded premature neonates or children admitted following surgery for congenital heart disease. | To explore the association of FB and mortality in children with sepsis and shock. | Small, heterogeneous sample. |
| On Cox regression, independent association between mortality and FO > 10% on D3 ICU (HR 7.27), when adjusted for illness severity, infection or malignancy. | ||||
| On logistic multivariable regression independent association between peak cumulative FB on D3 and D7 with mortality when adjusted for illness severity, presence of infection, VA use or time to ICU admission. | ||||
| D3 ICU FO > 10%: OR (mortality): 9.17 (95% CI: 2.22-55.57). | ||||
| Per day of FO: OR (mortality): 1.61 (95% CI: 1.21-2.28). | ||||
| Chen, et al. [96] 2016 | Retrospective single-centre observational study in a Chinese academic paediatric ICU. | 202 paediatric patients, 99% with severe sepsis within 24 h of admission, median age 0.5 (0.2-1.5) years, 53% male, with PIM II score of 4.2 (2.1-22) and 30.2% hospital mortality. Exclusions due to incomplete follow-up. | To explore the association of fluid accumulation and clinical outcomes in children with severe sepsis. | 20.3% developed FO ≥ 5%, 4.5% FO ≥ 10% within 24 h of admission, with 23.4% of those in ICU for > 48 h developing daily FO due to reduced output. |
| Independent association between FO ≥ 5% on D1 ICU and mortality when adjusted for age, antibiotics, MODS, and MV. | ||||
| FO ≥ 5% D1 ICU OR (mortality): 1.2 (95% CI: 1.08-1.33). | ||||
| FO% D1 ICU AUROC (mortality): 0.74 (95% CI: 0.65-0.82); 2.65% optimal cut-off. | ||||
| Paediatric ALI | ||||
| Flori, et al. [97] 2011 | Post-hoc analysis of a prospective multi-centre (2) observational study in US academic paediatric ICUs. | 313 paediatric patients with ALI, median age 3.4 (0-18) years, 56% male, all requiring respiratory support, with PRISM III score of 10.3 ± 8.7 and 21% hospital mortality. Exclusions due to LA HT, intracardiac shunt. | To explore the association of +FB and clinical outcomes in critically ill children with ALL | Possibly U-shaped relationship between +FB and mortality over the first 72 h when considered in 10 mL/kg/day increments. |
| Increasing FB independently associated with duration of MV, and independently associated with mortality, when adjusted for organ system failures. | ||||
| Per 10 mL/kg/day +FB: OR (mortality): 1.08 (95% CI: 1.01-1.15). | ||||
| Sinitsky, et al. [98] 2015 | Retrospective single-centre observational study in a UK academic paediatric ICU. | 636 paediatric patients, 59% with respiratory pathology, median age 1.05 (0.3-4.2) years, 57% male, all requiring respiratory support, with PIM II score of 6 (2-12) and 8% hospital mortality. Exclusions due to < 24 h MV, or no MV at 48h post-admission. | To explore the association of early FO and respirator/ outcomes in critically ill children. | No association demonstrated between FB-calculated FO% and mortality. |
| FO% at 48h was independently associated with oxygenation index when adjusted for diagnostic group, and with invasive ventilation days in survivors when adjusted for illness severity and diagnostic group. | ||||
| Detailed electronic nature of data amenable to further, more detailed modelling. | ||||
| Valentine, et al. [99] 2012 | Multi-centre (5) retrospective observational study from US academic paediatric ICUs. | 168 paediatric patients with ALI, 71% with direct pulmonary injury, median age 3 (0.8-11) years, 57% male, all requiring respiratory support, with PRISM III score of 9 (3-13) and 11.3% hospital mortality. Extensive exclusion criteria. | To explore the relationship between cumulative FB and ventilation, and the distributions of net and cumulative FB. | ICU D3 means cumulative intake 269 ± 203 mL/kg, FB 84 ± 93 mL/kg. |
| Independent association between D3 FB and ventilator-free days, but not mortality. Possibly related to sample size. | ||||
| Wilson, et al. [100] 2013 | Post-hoc analysis of multi-centre (24) RCT data from 6 countries (from the Calfactant in Acute Respiratory Distress Syndrome trial). | 109 paediatric patients, mean age 6.1 ± 5.8 years, 51% male, with PRISM III score of 11.4 ± 6.8 and 9.2% hospital mortality. Excluded moribund or palliative patients, patients with other organ failure or pre-existing lung disease, obtundation, or delayed recruitment. | To explore the association of FB and clinical outcomes in children with direct ALI. | Only represents a tiny fraction of patients admitted to ICUs, and atypical of those normally presenting with respiratory failure. |
| Significantly greater daily and cumulative FB ICU D1-7 in survivors. | ||||
| Only ≈ 10% of the variation in cumulative FB on ICU D1 and D7 explained by illness severity on multivariable modelling using age, gender, race, PRISM III score, initial oxygenation indices, type of lung injury, risk level, and treatment, none of which were independent predictors of FB. | ||||
Means are presented as mean ± standard deviation. Medians are presented as median (interquartile range). ≈ indicates estimate from information available, rather than direct calculation.
ICU: intensive care unit; APACHE: Acute Physiology and Chronic Health Evaluation scoring system; Cr: serum creatinine; FB: fluid balance; AKI: acute kidney injury; RRT: renal replacement therapy, implying filtration or continuous mode; CVD: cardiovascular disease; DM: diabetes mellitus; VA: vasoactive medication; CLD: chronic liver disease; MV: mechanical ventilation; OR: odds ratio; CI: confidence intervals; ICU D: day of ICU admission; FBT: fluid bolus therapy; BP: blood products; SAPS: Simplified Acute Physiology Score scoring system; ECMO: extracorporeal membrane oxygenation; ESRF: end-stage renal failure; FO: fluid overload; FO%: fluid overload percentage; CRRT: continuous renal replacement therapy; HR: hazard ratio; ICU ADM: total of ICU admission/to discharge; AUROC: area under the receiver operator curve; RCT: randomised controlled trial; TBI: traumatic brain injury; CVP: central venous pressure; UO: urine output; IVF: intravenous fluid; G: group; SOFA: Sequential Organ Failure Assessment scoring system; EGDT: early goal directed therapy; CKD: chronic kidney disease; CHF: chronic heart failure; IHD: ischaemic heart disease; ARDS: acute respiratory distress syndrome; NIV: non-invasive ventilation; CHD: chronic haemodialysis; UV: urine volume; PDA: patent ductus arteriosus; PIM: Paediatric Index of Mortality scoring system; PICU: paediatric intensive care unit; GFR: glomerular filtration rate; LOS: length of stay; PRISM: Paediatric Risk of Mortality scoring system; PAC: pulmonary artery catheter; CVC: central venous catheter; ALI: acute lung injury; LA HT: left atrial hypertension.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download