Abstract
Background
The effectiveness of surveillance to identify extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-E) carriers is controversial during a non-outbreak situation. We performed additional stool cultures for ESBL-E among intensive care unit (ICU) patients already under active surveillance by means of sputum and urine cultures. We aimed to assess the efficacy of stool cultures for screening for ESBL-E in a non-outbreak situation.
Methods
We conducted a retrospective cohort study in an ICU. Sputum and urine samples were cultured for ESBL-E surveillance purposes from January to September 2013 (phase 1). Stool cultures were routinely performed in addition from January to September 2014 (phase 2). Antimicrobial use density values and clinical outcomes were investigated and compared between phase 1 and 2.
Results
We identified 512 and 478 patients in phase 1 and phase 2, respectively. ESBL-E were found in the feces of 65 (13.6%) patients in phase 2. The antimicrobial use density values (expressed as defined daily doses per 1,000 bed-days) were not significantly different between the two phases for fluoroquinolones (7 vs. 10, p = 0.376), third-generation cephalosporins (24.2 vs. 29.5, p = 0.724), tazobactam/ piperacillin (44.6 vs. 57.3, p = 0.489), and carbapenems (73 vs. 55.5, p = 0.222). Moreover, there were no significant differences in ICU mortality and length of stay (11.5% vs. 9.8%, p = 0.412, and 9 vs. 10 days, p = 0.28, respectively).
Extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-E) are important because few antibiotics are effective against these pathogens. Moreover, clinical outcomes of patients with ESBL-E infections are poorer, with higher mortality and decreased clinical response rates compared with susceptible Enterobacteriaceae.[1] However, the effectiveness of surveillance for ESBL-E carriage, particularly in the absence of an outbreak, remains controversial. Past studies showed that the effectiveness of surveillance for preventing ESBL-E cross-transmission was limited.[2,3] However, other studies showed prior colonization with an ESBL-E to be a risk factor for subsequent ESBL infections.[4,5] Hence, some hospitals use culture-based methods for active surveillance of antimicrobial drug-resistant gram-negative bacteria to help guiding empiric antibiotic therapy.[6]
In December 2008, a nosocomial outbreak of multidrug-resistant Acinetobacter baumannii occurred in an intensive care unit (ICU) at our institution. During this outbreak, we began performing active surveillance cultures of sputum and urine, together with enforcing contact precautions to control and monitor antimicrobial drug-resistant bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA), multidrug-resistant Pseudomonas aeruginosa (MDRP), multidrug-resistant Acinetobacter baumannii (MDRAB), carbapenem-resistant Enterobacteriaceae (CRE), and ESBL-E. This surveillance has continued even after the outbreak was controlled. In addition, pilot surveillance for ESBL-E carriage by means of stool cultures was conducted over a 9-month period. We therefore conducted a retrospective cohort study to evaluate the clinical influence of this surveillance strategy during a non-outbreak situation in an adult ICU population.
This retrospective, single-center, observational study was conducted in the Department of Emergency and Critical Care Medicine of a tertiary-level hospital in Japan. The hospital has 34 ICU beds and receives 800 ICU admissions per year in average. All patients older than 18 years old admitted to the ICU for longer than 24 hours were enrolled. This study was approved by the institutional review board in our hospital, according to the Declaration of Helsinki (number 16-6-09). The study periods were consisted of two phases: January to September 2013 (phase 1); control phase and January to September 2014 (phase 2); surveillance phase. The stool surveillance for ESBL-E carriage was conducted in phase 2. Both phases were set in the same timing of two consecutive years to avoid confounding effects of seasonal variation. Contact precautions were used for all patients during both phases of this study. In both phases, all patients had active surveillance cultures of sputum and urine specimens performed at ICU admission, and twice weekly until ICU discharge. In addition, stool cultures were analyzed at ICU admission and twice weekly thereafter in phase 2 to determine ESBL-E fecal colonization of patients. Patients with chronic constipation had surveillance cultures performed as regularly as possible without using laxatives. Patients whose culture results showed ESBL-E colonization did not receive isolation precautions during the study period.
The medical records were reviewed to investigate patient characteristics. Surveillance-culture data, antimicrobial use density (AUD) values, the incidence of ESBL-E infections, the initial empirical antibiotic choice for patients with ESBL-E infections, ICU mortality, and ICU length of stay (LOS) for the described periods were evaluated. These parameters were compared between phase 1 and phase 2. Moreover, we grouped the patients of phase 2 into ESBL-E carriers and non-carriers, and the incidence of ESBL-E infections, ICU mortality rates, and ICU LOS were compared between these two groups.
To screen for ESBL production, all cultures were performed on selective media (CHROMagarTM ESBL, Kanto Chemical Co., Tokyo, Japan). Isolated bacteria were cultured and subjected to identification and antimicrobial susceptibility testing using the VITEK-2® automated microbiology system (Sysmex-bioMerieux, Tokyo, Japan). In addition, double-disk synergy tests (cefotaxime, ceftazidime, and cefpodoxime vs. clavulanic acid) were also performed according to the guidelines provided by the Clinical and Laboratory Standards Institute.[7] We obtained data for all intravenous antimicrobial administrations from the electronic medical records. The quantity of prescribed antimicrobial agents was expressed as the defined daily dose (DDD) and AUD. The DDD is defined by the World Health Organization as the assumed mean maintenance adult daily dose of an antimicrobial agent for one day of treatment.[8] The AUD was expressed as the DDD per 1,000 patient-days for individual antimicrobial agents. This was calculated and the quantities of the following antimicrobials used in our department were compared: fluoroquinolones, third-generation cephalosporins, tazobactam/piperacillin, and carbapenems.
All statistical analyses were performed using the EZR software program (Saitama Medical Center, Jichi Medical University, Saitama, Japan),[9] which is a graphical user interface for the R software program (The R Foundation for Statistical Computing, Vienna, Austria). Continuous data were expressed as the median and interquartile range. Statistical differences between two groups were determined using the Mann–Whitney U-test or the chi-squared test, as appropriate. All tests were two-tailed, and p values of <0.05 were considered statistically significant.
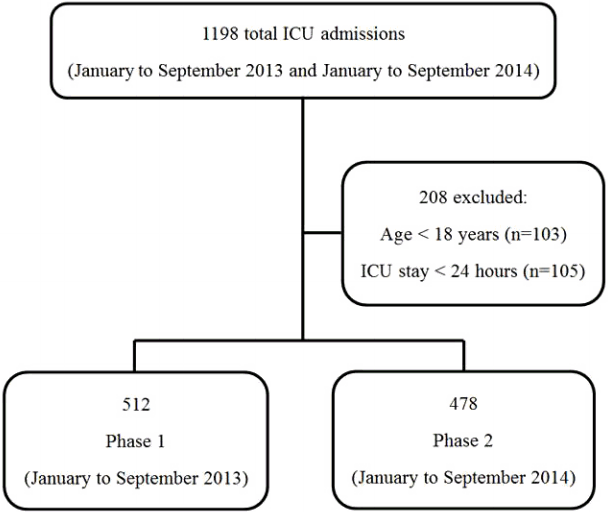
We identified 512 and 478 patients in phases 1 and 2, respectively (Fig. 1). The comparison of the background of patients and the detection rate of surveillance cultures for MRSA, MDRP, MDRAB, CRE, and ESBL-E in each phase are shown in Table 1. By cultures from sputum and urine samples, MDRP, MDRAB, and CRE were not detected in neither phases and the detection rate of MRSA and ESBL-E was not significantly different between two phases. ESBL-E were found in the feces of 65 of 478 (13.6%) patients in phase 2. The following ESBL-producing organisms were isolated from the stool cultures: Escherichia coli (n = 41), Klebsiella pneumoniae (n = 25), and Enterobacter aerogenes (n = 2). The AUD values for the following antibiotics were not significantly different between two phases: fluoroquinolones (7 vs. 10, p = 0.376), third-generation cephalosporins (24.2 vs. 29.5, p = 0.724), tazobactam/piperacillin (44.6 vs. 57.3, p = 0.489), and carbapenems (73 vs. 55.5, p = 0.222). In addition, the incidence of ESBL-E infections (0.6% vs. 0.8%, p = 0.717), ICU mortality (11.5% vs. 9.8%, p = 0.412), and ICU LOS (9 days vs. 10 days, p = 0.28) were not significantly different between two phases of the study (Table 2). There was no significant difference in the initial empiric antibiotic choice for patients with ESBL-E infections between two phases (Table 3).
The comparison between ESBL-E carriers and noncarriers in phase 2 is shown in Table 4. The incidence of ESBL-E infections among ESBL-E carriers and ICU LOS were significantly higher and longer than among non-carriers (4.6% vs. 0.2%, p = 0.009, and 18.5 days vs. 9 days, p < 0.001, respectively), but ICU mortality (9.2% vs. 9.9%, p = 1.000) was not different.
In this retrospective study, we attempted to evaluate the clinical benefit, which included evidence of improvement in the AUD of broad-spectrum antimicrobials, clinical patient outcomes, and ICU LOS, of routine stool cultures for ESBL-producing Enterobacteriaceae in ICU patients during a non-outbreak situation. The presence of ESBL-E in the gastrointestinal tract is usually harmless to the host. However, ESBL-E may cause infection in the carrier in certain situations, such as immunosuppression, gastrointestinal surgery, or physical debilitation. Surveillance information about ESBL-E carriage can help to identify which patients should receive empiric ESBL-targeted antimicrobial therapy. Since carbapenems are the primary therapeutic option for severe infections caused by ESBL-producing bacteria[10], we hypothesized that carbapenems might actively be selected for empiric treatment of patients with sepsis known to have intestinal ESBL-E colonization. However, the AUD values for carbapenems were not significantly different between the two phases of this study, and the frequency of carbapenem use for empiric treatment of patients with ESBL-E infections, also, was not different between the two phases. Thus, the decision to use carbapenems for empiric treatment of sepsis may be made according to the patient’s clinical severity, and might not be influenced by ESBL-E carriage surveillance information. Furthermore, the information of ESBL-E carriage surveillance had no benefit in terms of improving ICU mortality and ICU LOS in the present study.
The present study showed that the prevalence of gastrointestinal carriage of ESBL-E in ICU patients during a non-outbreak situation was 13.6%. ESBL-E colonization rates among ICU patients range from 2.2% in the Unites States[11] to 49.0% in India,[12] with geographical differences observed globally.[13] Previous Japanese studies have reported the prevalence of ESBL-E carriage to be 6.4% in healthy adults,[14] and 12% in pediatric tertiary care hospital patients.[15] In addition, a study in Spain reported that the prevalence of ESBL-E carriage was 3.7% in healthy volunteers, 5.5% in outpatients, and 12% in hospitalized patients.[16] As already reported, previous antibiotic use is a major risk factor for rectal carriage of ESBL-producing bacteria.[17,18] Thus, the prevalence of ESBL-E carriage may be higher in hospitalized patients, as they are more likely to have received antibiotic therapy.
ICU mortality did not differ significantly between ESBL-E carriers and non-carriers in phase 2. However, the incidence of ESBL-E infections among ESBL-E carriers and ICU LOS were significantly higher and longer than among non-carriers. Several studies have shown that a long hospital stay is one of the risk factors for ESBL-E colonization[12,18,19]; this was reflected in the present study findings. Furthermore, previous studies reported colonization with ESBL-E was a risk factor for subsequent ESBL infections[2,5] and that the ESBL infections rates in colonized ICU patients ranged from 4.9%[19] to 68.8%[20]; the results of the present study are in accordance with these previous studies. Another study reported that carriage of ESBL-E persisted for 12 months after having had an infection.[21] Thus, although intestinal ESBL-E colonization surveillance did not influence the clinical decision-making or outcomes in this study, it may be useful for treatment decisions for subsequent infections in ESBL-E carriers (for example, subsequent hospitalization for urosepsis). Further studies are needed to confirm the effects of active surveillance for ESBL-E carriage in several situations.
The present study has some limitations. First, it was a retrospective study involving a single center with a small sample size. Second, because surveillance of ESBL-E carriage was performed using noninvasive procedures, we could not collect specimens at a fixed interval using rectal swab cultures. Furthermore, we were unable to evaluate the transmission rate, bacterial strains, or ESBL genes.
In conclusion, our study demonstrated that the performance of routine stool cultures for ESBL-E in ICU patients during a non-outbreak situation seemed to be ineffective in improving the AUD of broad-spectrum antimicrobials, clinical patient outcomes, and ICU LOS. Further studies are needed to determine the efficacy of active surveillance for intestinal colonization with ESBL-E.
References
1. Tumbarello M, Spanu T, Sanguinetti M, Citton R, Montuori E, Leone F, et al. Bloodstream infections caused by extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae: risk factors, molecular epidemiology, and clinical outcome. Antimicrob Agents Cemother. 2006; 50:498–504.
2. Thouverez M, Talon D, Bertrand X. Control of enterobacteriaceae producing extended-spectrum beta-lactamase in intensive care units: rectal screening may not be needed in non-epidemic situations. Infec Control Hosp Epidemiol. 2004; 25:838–41.

3. Han JH, Bilker WB, Nachamkin I, Zaoutis TE, Coffin SE, Linkin DR, et al. The effect of a hospitalwide urine culture screening intervention on the incidence of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella species. Infec Control Hosp Epidemiol. 2013; 34:1160–6.
4. Troché G, Joly LM, Guibert M, Zazzo JF. Detection and treatment of antibiotic-resistant bacterial carriage in a surgical intensive care unit: a 6-year prospective survey. Infect Control Hosp Epidemiol. 2005; 26:161–5.

5. Liss BJ, Vehreschild JJ, Cornely OA, Hallek M, Fätkenheuer G, Wisplinghoff H, et al. Intestinal colonisation and blood stream infections due to vancomycin-resistant enterococci (VRE) and extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBLE) in patients with haematological and oncological malignancies. Infection. 2012; 40:613–9.

6. Blot S, Depuydt P, Vogelaers D, Decruyenaere J, De Waele J, Hoste E, et al. Colonization status and appropriate antibiotic therapy for nosocomial bacteremia caused by antibiotic-resistant gram-negative bacteria in an intensive care unit. Infect Control Hosp Epidemiol. 2005; 26:575–9.

7. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; Nineteenth informational supplement. CLSI document M100-S19. Wayne: Clinical and Laboratory Standards Institute;2009.
8. The WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment 2016 [Internet]. Oslo: WHO Collaborating Centre for Drug Statistics Methodology;c2016. [accessed on 20 May 2016]. Available from: http://www.whocc.no/atcddd/.
9. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013; 48:452–8.

10. Tamma PD, Han JH, Rock C, Harris AD, Lautenbach E, Hsu AJ, et al. Carbapenem therapy is associated with improved survival compared to piperacillin-tazobactam for patients with extended-spectrum β-lactamase bacteremia. Clin Infect Dis. 2015; 60:1319–25.
11. Harris AD, McGregor JC, Johnson JA, Strauss SM, Moore AC, Standiford HC, et al. Risk factors for colonization with extended-spectrum β-lactamase-producing bacteria and intensive care unit admission. Emerg Infect Dis. 2007; 13:1144–9.

12. Azim A, Dwivedi M, Rao PB, Baronia AK, Singh RK, Prasad KN, et al. Epidemiology of bacterial colonization at intensive care unit admission with emphasis on extended-spectrum beta-lactamase- and metallo-beta-lactamase-producing Gram-negative bacteria--an Indian experience. J Med Microbiol. 2010; 59(Pt 8):955–60.
13. Sidler JA, Battegay M, Tschudin-Sutter S, Widmer AF, Weisser M. Enterococci, Clostridium difficile and ESBL-producing bacteria: epidemiology, clinical impact and prevention in ICU patients. Swiss Med Wkly. 2014; 144:w14009.

14. Luvsansharav UO, Hirai I, Niki M, Nakata A, Yoshinaga A, Moriyama T, et al. Prevalence of fecal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae among healthy adult people in Japan. J Infect Chemother. 2011; 17:722–5.

15. Minami K, Shoji Y, Kasai M, Ogiso Y, Nakamura T, Kawakami Y, et al. Proportion of rectal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae in the inpatients of a pediatric tertiary care hospital in Japan. Jpn J Infect Dis. 2012; 65:548–50.
16. Valverde A, Coque TM, Sánchez-Moreno MP, Rollán A, Baquero F, Cantón R. Dramatic increase in prevalence of fecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae during nonoutbreak situations in Spain. J Clin Microbiol. 2004; 42:4769–75.
17. Malloy AM, Campos JM. Extended-spectrum beta-lactamases: a brief clinical update. Pediatr Infect Dis J. 2011; 30:1092–3.
18. Ajao AO, Johnson JK, Harris AD, Zhan M, McGregor JC, Thom KA, et al. Risk of acquiring extended-spectrum β-lactamase-producing Klebsiella species and Escherichia coli from prior room occupants in the intensive care unit. Infect Control Hosp Epidemiol. 2013; 34:453–8.
19. Razazi K, Derde LP, Verachten M, Legrand P, Lesprit P, Brun-Buisson C. Clinical impact and risk factors for colonization with extended-spectrum β-lactamase-producing bacteria in the intensive care unit. Intensive Care Med. 2012; 38:1769–78.

20. Christiaens G, Ciccarella Y, Damas P, Hayette MP, Melin P, Nys M, et al. Prospective survey of digestive tract colonization with enterobacteriaceae that produce extended-spectrum beta-lactamases in intensive care units. J Hosp Infect. 2006; 62:386–8.
Table 1.
Demographic characteristics and surveillance-culture data
| Variable | Phase 1 (n = 512) | Phase 2 (n = 478) | p-value |
|---|---|---|---|
| Age (years) | 64 (47-76) | 67 (53-77) | 0.051 |
| Sex (M/F) | 295/217 | 301/177 | 0.091 |
| APACHE II score | 16 (11-22) | 17 (12-22) | 0.268 |
| Hemodialysis | 51 (10.0) | 45 (9.4) | 0.830 |
| Mechanical ventilation | 209 (40.3) | 182 (38.1) | 0.476 |
| Admission category | |||
| Trauma | 138 (27.0) | 108 (22.6) | 0.122 |
| Stroke | 126 (24.6) | 136 (28.5) | 0.172 |
| Sepsis* | 56 (10.9) | 59 (12.3) | 0.552 |
| Cardiovascular | 48 (9.4) | 54 (11.3) | 0.347 |
| Surgical | 27 (5.3) | 29 (6.1) | 0.680 |
| Drug intoxication | 23 (4.5) | 20 (4.2) | 0.877 |
| Other | 94 (18.4) | 72 (15.1) | 0.174 |
| Cultures positive for MRSA | |||
| Sputum | 51 (10.0) | 56 (11.7) | 0.413 |
| Urine | 13 (2.5) | 8 (1.7) | 0.384 |
| Cultures positive for MDRP† | |||
| Sputum | 0 | 0 | - |
| Urine | 0 | 0 | - |
| Cultures positive for MDRAB† | |||
| Sputum | 0 | 0 | - |
| Urine | 0 | 0 | - |
| Cultures positive for CRE | |||
| Sputum | 0 | 0 | - |
| Urine | 0 | 0 | - |
| Cultures positive for ESBL-E | |||
| Sputum | 6 (1.2) | 11 (2.3) | 0.222 |
| Urine | 18 (3.5) | 20 (4.2) | 0.622 |
| Stool | 0 | 65 (13.6) | - |
Data are presented as n (%) or median (interquartile range).
M: male; F: female; APACHE: acute physiology and chronic health evaluation; MRSA: methicillin-resistant Staphylococcus aureus; MDRP: multidrug-resistant Pseudomonas aeruginosa; MDRAB: multidrug-resistant Acinetobacter baumannii; CRE: carbapenem-resistant Enterobacteriaceae; ESBL-E: extended-spectrum betalactamase-producing Enterobacteriaceae.
Table 2.
Comparison of clinical variables in each sub-phase
Table 3.
Initial empiric antibiotic choice for patients with ESBL-E infections in each sub-phase
| Variable | Phase 1 (n = 3) | Phase 2 (n = 4) |
|---|---|---|
| Third-generation cephalosporins | 1 (33.3) | 1 (25.0) |
| Tazobactam/piperacillin | 1 (33.3) | 1 (25.0) |
| Carbapenems | 1 (33.3) | 2 (50.0) |
Table 4.
Comparison of clinical variables between ESBL-E carriers and non-carriers in phase 2
| Variable | Carriers (n = 65) | Non-carriers (n = 413) | p-value |
|---|---|---|---|
| Incidence of ESBL-E infections | |||
| Total | 3 (4.6) | 1 (0.2) | 0.009* |
| Pneumonia | 1 (1.5) | 0 | - |
| Urinary tract infection | 2 (3.1) | 0 | - |
| Peritonitis | 0 | 1 (0.2) | - |
| ICU mortality | 6 (9.2) | 41 (9.9) | 1.000 |
| ICU LOS (days) | 18.5 (11.3-32.5) | 9 (4.0-18.0) | < 0.001* |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download