A 36-year-old primigravida woman with a twin pregnancy by in vitro fertilization was admitted to the labor and delivery service at our hospital. She had suffered from gestational hypertension and diabetes mellitus during the pregnancy. Laboratory findings, including complete blood count (white blood cell 6,410 cells/mm
3, hematocrit 32.7%, platelets 247 × 10
3/mm
3), liver function test, electrolytes (blood urea nitrogen [BUN] 5.0 mg/dL, creatinine 0.56 mg/dL, sodium 140 mEq/L, chloride 110 mEq/L, potassium 3.8 mEq/L, glucose 90 mg/dL), and coagulation profile (prothrombin time 10.9 seconds, international normalized ratio [INR] 1.01, activated prothrombin time 24.9 seconds) were within normal limits. Chest radiography and electrocardiography were not remarkable. Because of an uncontrollable progression of labor, the patient underwent an emergency low-transverse cesarean section at 30 weeks and 5 days of gestation under general anesthesia. She delivered twin babies without any adverse events. Upon completion of surgery, she was extubated and was awake and alert. Blood pressure was 140/110 mmHg and heart rate 110 beats/minute. During the surgery, 2800 mL of crystalloids and 600 mL of colloid were administered. Intraoperative blood loss was 600 mL, and urine output was 500 mL. Shortly after extubation, she suddenly became breathless and cyanotic. Oxygen saturation according to pulse oximetry was 75% and decreased to 40%. She was reintubated promptly and placed on mechanical ventilation with 100% oxygen. SpO
2 increased to 90%. The end-tidal PCO
2 was 15 mmHg initially and then decreased to zero. She also developed hypotension and bradycardia, and her heart stopped beating. Only 7 or 8 minutes elapsed between the onset of dyspnea and cardiac arrest, despite the effort to resuscitate her with an immediate intervention. Cardiopulmonary resuscitation was started, along with infusion of vasopressors and inotropic agents. Veno-arterial ECMO, which is essentially a heart-lung machine without a reservoir, providing both respiratory and circulatory support, was applied immediately via the femoral vein and artery after 3,000 units of heparin were administered. After vessel puncture and guide-wire placement, venous access with a 28-French cannula was directed through the right femoral vein to the right atrium under transthoracic echocardiographic guidance, and arterial access with a 16-French cannula was introduced into the left femoral artery. A CAPIOX
® EBS circuit (Terumo Inc., Tokyo, Japan) and CAPIOX
® SP pump console SP-101 (Terumo Inc., Tokyo, Japan) were used. The initial setting of the ECMO included a blood flow of 6.0 L/minute and FiO
2 of 0.75. With the initiation of ECMO, 20 minutes after cardiopulmonary arrest, the patient’s vital signs became stable (blood pressure was 149/106 mmHg, heart rate 115 beats/minute, and SpO
2 100%), and most of the vasoactive medications were tapered gradually. The patient was sent for chest computed tomography (CT) angiography to rule out other major problems than amniotic fluid embolism before being transferred to the intensive care unit. Chest CT angiography revealed no pulmonary embolism, but intense parenchymal opacification in the bilateral-dependent lung and nondependent ground-glass opacification were apparent (
Fig. 1). Transthoracic echocardiography revealed left ventricular global hypokinesia with severe left ventricular dysfunction (
Fig. 2). There was a significant amount of vaginal bleeding; hematocrit was 26.9%, and platelet count was 72,000/mm
3. Prothrombin time was 16.9 seconds, INR 1.61, and activated thromboplastin time 63.0 seconds.
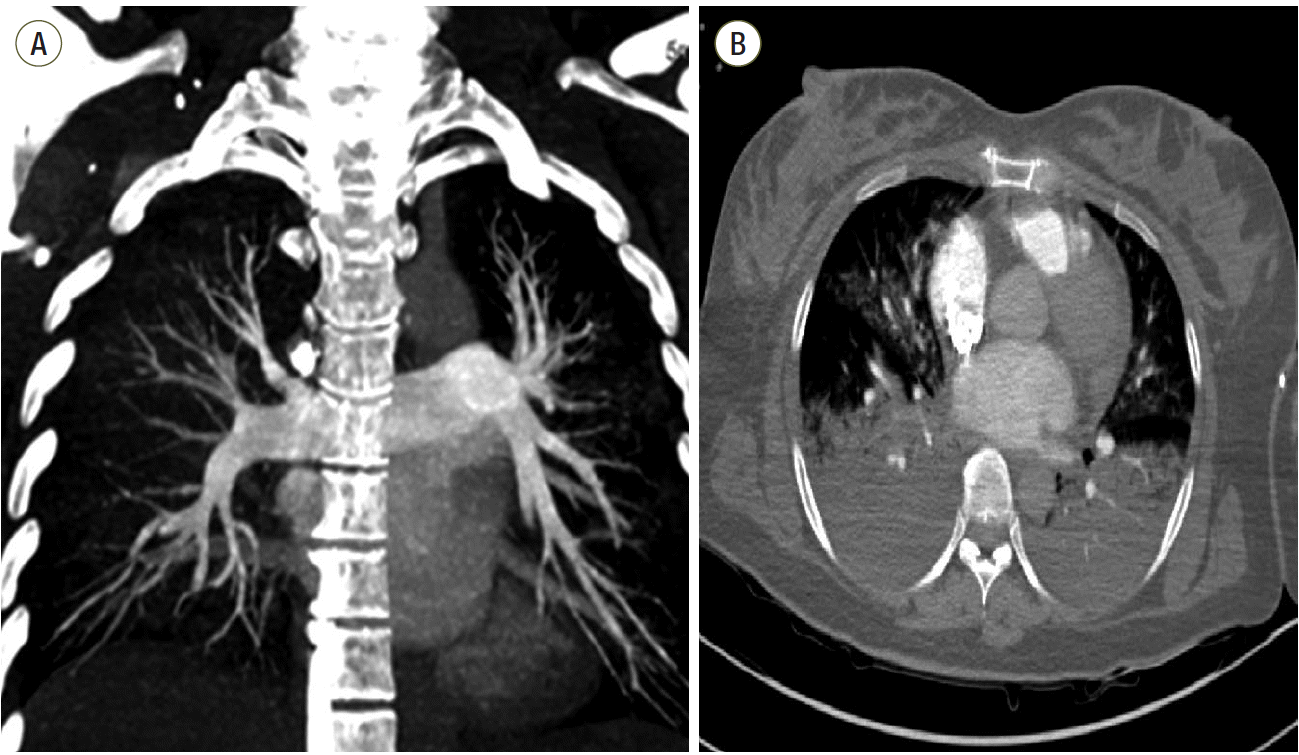 | Fig. 1.Computed tomography (CT) scans from a 36-year-old female who survived acute respiratory distress syndrome. (a) CT pulmonary angiography with coronal maximum intensity projection reconstruction reveals no filling defect. (b) Axial CT scan with lung algorithm
displays typical areas of intense parenchymal opacification in the dependent lung and nondependent ground-glass opacification. 
|
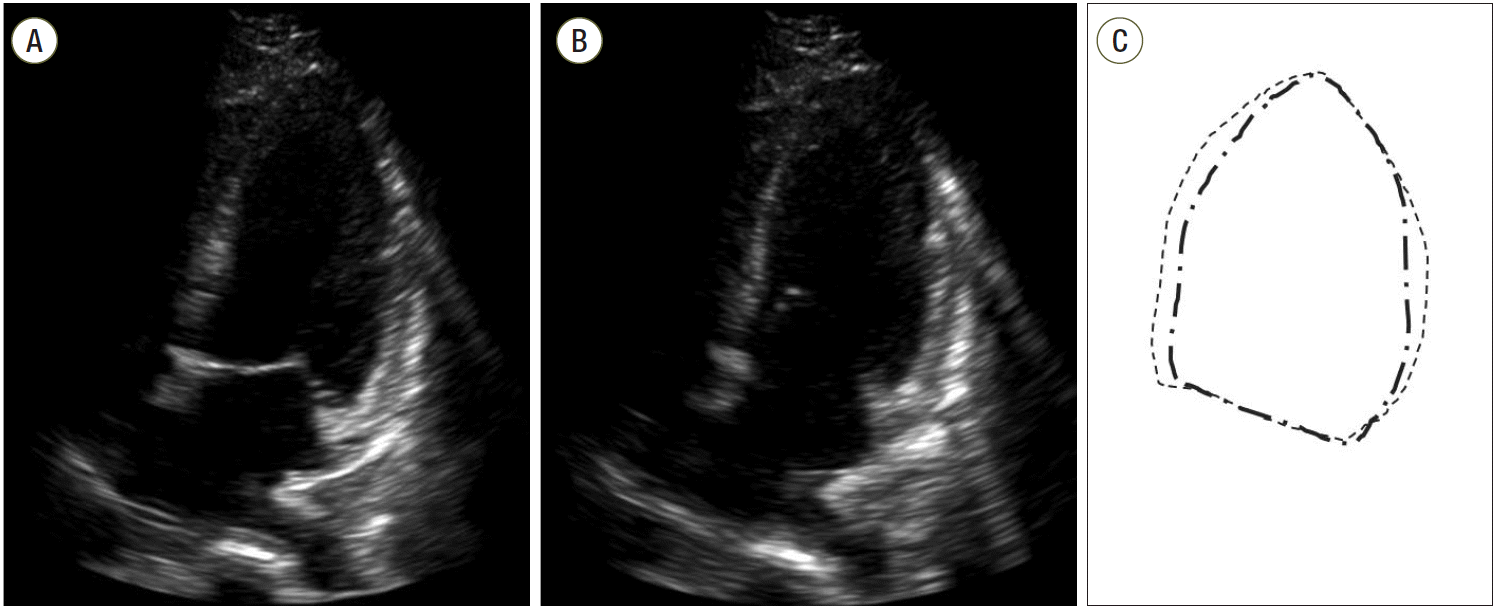 | Fig. 2.Echocardiograms (A, B) and schematic representation of the left ventricular (LV) echocardial borders (C) during diastole and systole. Transthoracic 4–chamber view at admission demonstrates apical and midventricular dilatation of the LV, accompanied by severe
global hypokinesia. 
|
On the first day in the intensive care unit (ICU), the patient was hemodynamically stable with an ECMO blood flow rate of 4.6 L/minute. Urine output had been greater than 1 mL/kg of body weight/hour. The lactate level was less than 4 mEq/L. Arterial blood gases while on an FiO
2 of 0.6 with ECMO indicated that pH was 7.43, PCO
2 29 mmHg, PO
2 166 mmHg, and HCO
3- 19.2 mEq/L. Fibrin degradation product was 58.3 mcg/mL, D-dimer 24.8 mcg/L, and fibrinogen 194 mg/dL. For the evaluation of hemodynamic functions, including the degree of pulmonary vasoconstriction and left ventricular filling pressure, a pulmonary artery catheter was introduced through the right internal jugular vein. Initially, mean pulmonary arterial pressure and pulmonary capillary wedge pressure were 40 mmHg and 18 mmHg, respectively, and these slowly normalized over time. A blood sample from the pulmonary artery catheter with the balloon inflated was sent to pathology for cytological analysis. The result a few days later revealed the presence of degenerated squamous cells, indicating amniotic fluid embolism (
Fig. 3). On the patient’s second day in the ICU, follow-up echocardiography revealed nearly normalized left ventricular systolic function. Analysis of arterial blood gases while she was on the mechanical ventilator with a positive end-expiratory pressure of 5 cm H
2O and an FiO
2 of 0.4 indicated that pH was 7.45, PCO
2 33 mmHg, PO
2 143 mmHg, and HCO
3- 22.9 mEq/L. Hematocrit was 28.9%, and platelet count 140 × 10
3/mm
3. BUN was 27.0 mg/dL, and creatinine 0.88 mg/dL. On the third day in the ICU, the patient was weaned off ECMO, and a chest radiograph revealed a reduced extent of consolidation and improved aeration in both lungs (
Fig. 4). On the patient’s seventh day in the ICU, a tracheostomy was performed in anticipation of prolonged ventilatory support and airway problems because of an altered mental status, which is one of the cardinal findings of amniotic fluid embolism. Although the patient was awake, she was unable to obey verbal commands. On the 11th day in the ICU, she was weaned off the ventilator and transferred to the general ward. On the 40th hospital day, she was discharged with impaired cognitive function to a nursing home. And premature twin babies who were transferred to neonatal ICU for care of incubator were discharged safely with mother in healthy state.
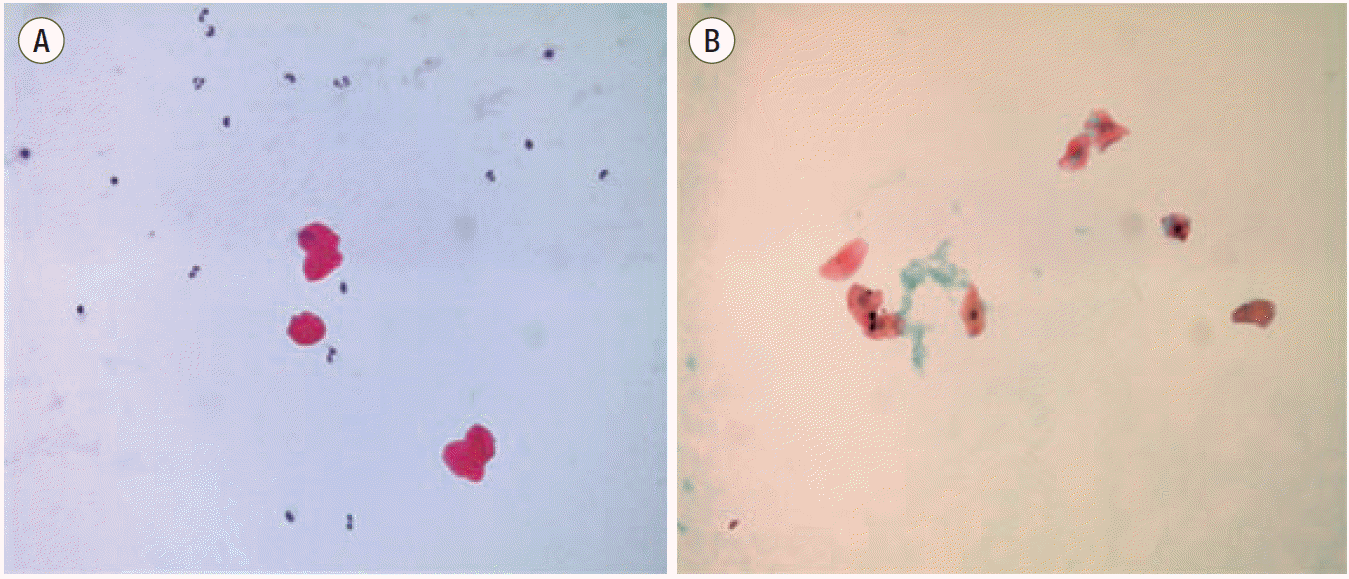 | Fig. 3.Pathology of a central venous blood sample reveals degenerative squamous epithelium (A: Hematoxylin and eosin stain, B: Papanicolaou stain). 
|
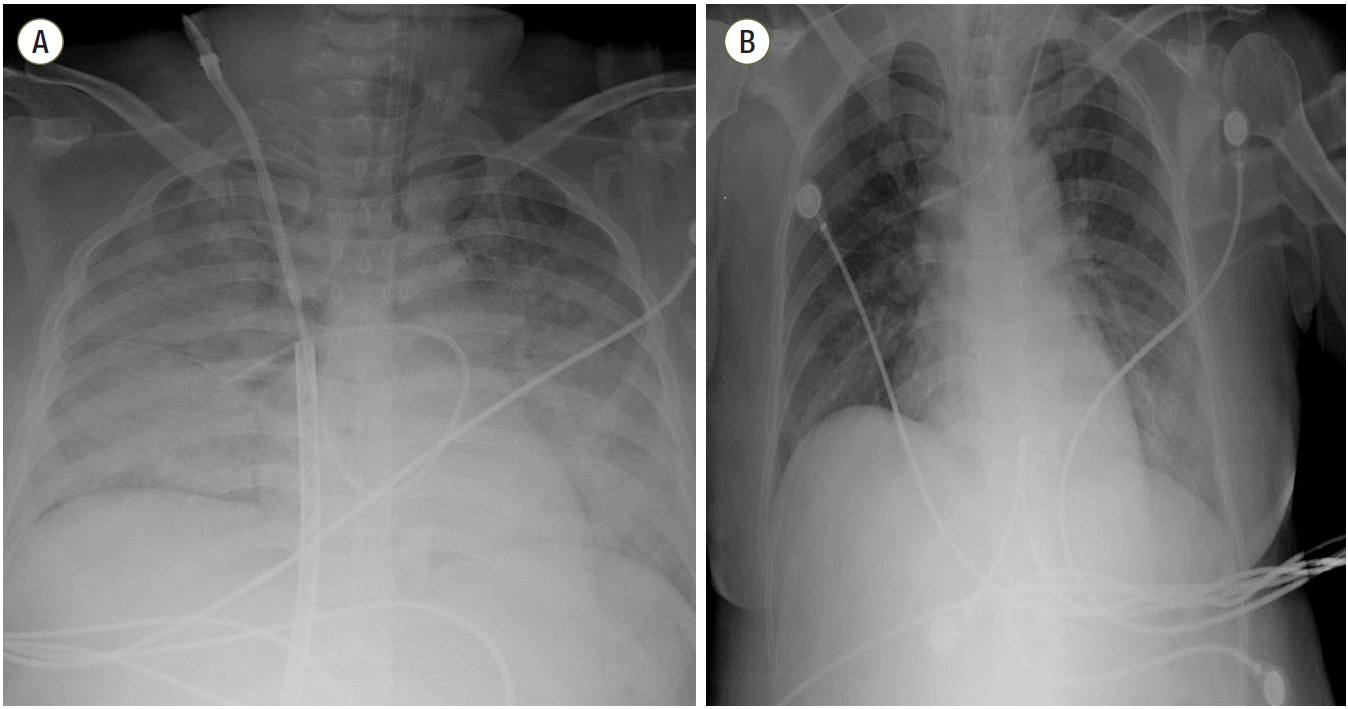 | Fig. 4.Chest radiograph from a 36-year-old female who survived acute respiratory distress syndrome. (A) Initial chest radiograph displays typical areas of diffuse consolidation in both lungs. The patient required extracorporeal membrane oxygenation (ECMO) due to uncorrected desaturation. (B) After 1 week, the ECMO catheter was removed. The chest radiograph displays the reduced extent of consolidation and improved aeration in both lungs. 
|







 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download