This article has been corrected. See "Spontaneous Lumbar Artery Bleeding and Retroperitoneal Hematoma in a Patient Treated with Continuous Renal Replacement Therapy" in Volume 31 on page 71.
Abstract
Rupture of the lumbar artery is usually associated with trauma but rarely has been reported in association with anticoagulation. We present a 71-year-old man who developed spontaneous rupture of the lumbar artery leading to a retroperitoneal hematoma while receiving continuous renal replacement therapy (CRRT). The bleeding was confirmed by computed tomography and angiography and was controlled successfully using selective angiographic embolization. We suggest that spontaneous retroperitoneal bleeding should be considered in a case of sudden decrease in hemoglobin in a CRRT patient.
Go to : 
Retroperitoneal bleeding secondary to lumbar artery injury is typically associated with trauma or surgery.[1] Spontaneous lumbar artery rupture is rare and may be related to vascular lesions, coagulopathy or anticoagulation therapy.[2,3]
The main advantage of continuous renal replacement therapy (CRRT) is the slower rate of solute or fluid removal per unit of time, but one requirement of CRRT that has potential deleterious consequences is anticoagulation.
We report a case of retroperitoneal hematoma due to spontaneous rupture of the lumbar artery in a patient treated with CRRT for severe edema and review the different methods of anticoagulation employed during CRRT.
A 71-year-old male patient was transferred to our hospital due to generalized edema and dyspnea. He had a long history of type II diabetes mellitus and had recently developed severe edema; related weight gain was over 10 kg. On physical examination, he had pale conjunctivae and pretibial pitting edema. Inspiratory crackle was heard on both lower lung fields. On admission, he had a pulse of 80 beats/min, a respiratory rate of 20 breaths/min, and blood pressure of 130/70 mmHg. Laboratory results included hemoglobin, 8.0 g/dL; white blood cell count, 5,530/mm3; platelet count, 267,000/mm3; creatinine 2.8 mg/dL; albumin, 1.2 g/dL; prothrombin time (PT), 10.9 sec; activated partial thromboplastin time (aPTT), 26.9 sec; sodium, 141 mEq/L; potassium, 3.9 mEq/L; and 24-hr urine protein, 5.6 g/day. Blood gas analysis revealed a pH of 7.39, PaCO2 of 30.3 mmHg, PaO2 of 77.5 mmHg, HCO3- of 18.3 mmol/L, and SaO2 of 95.6%. Echocardiography demonstrated concentric left ventricular hypertrophy with impaired relaxation and increased intima-media thickness in the common carotid artery.
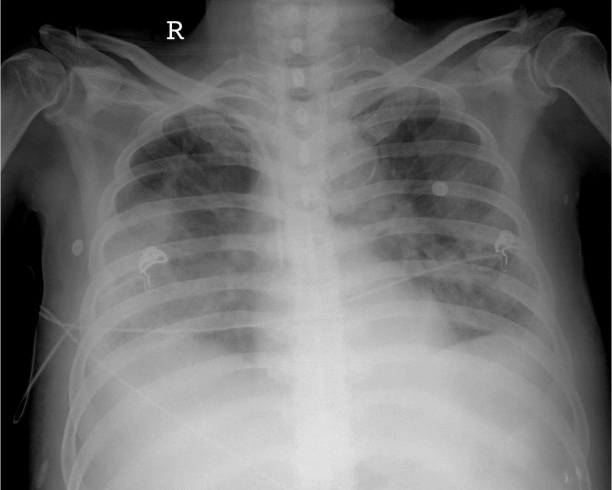
We administered intravenous furosemide, but edema and pleural effusion worsened (Fig. 1). On day 5 after admission, the patient was transferred to the intensive care unit (ICU) and CRRT was performed. The treatment settings were blood flow, 120 mL/min; dialysate flow, 1,000 mL/h; replacement fluid flow, 1,000 mL/h; and ultrafiltration rate, 200-300 mL/h. Heparin was administered as a bolus of 2,000 international units (IU), followed by continuous infusion of 600 IU/h into the dialysis circuit.
On day 8 after admission, the 3rd day of CRRT, the patient exhibited a change in mental status. His blood pressure was 60/40 mmHg and pulse rate was 114 beats/min. Hemoglobin fell to 4.4 g/dL. PT and PTT were within normal range (13 sec/32.4 sec) and his platelet count was 147,000/mm3. Under suspicion of internal bleeding and hemorrhagic shock, we administered intravenous fluids, blood transfusion, and oxygen supplementation; we also injected erythropoietin.
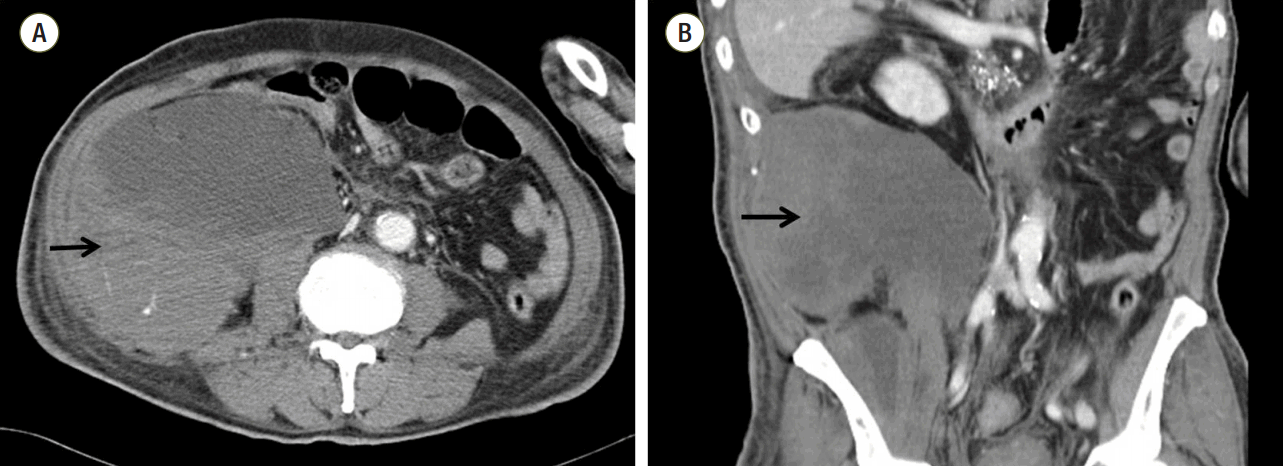
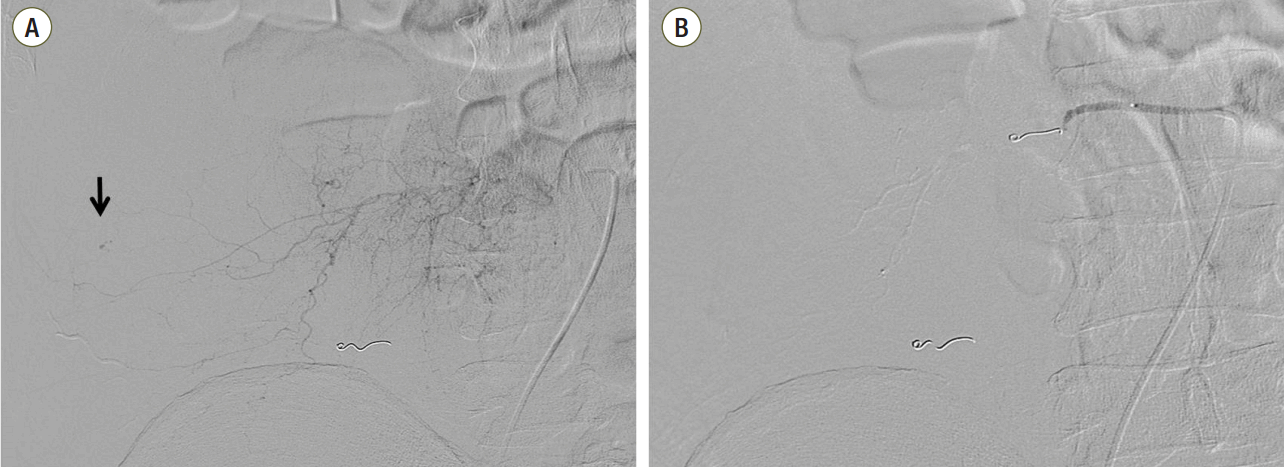
The patient underwent abdominal computed tomography, which showed a large hematoma in the right retroperitoneum and the right iliacus muscle (Fig. 2). For diagnostic and therapeutic purposes, the patient underwent emergent angiography. Contrast leakage was found in the right lumbar artery. Endovascular embolization with gelfoam® (Cali-Gel, Hangzhou Alicon Pharm SCI. & TEC. Co., Ltd., Hangzhou City, Zhejiang, China) and microcoils (Tornado, Cook Medical Inc., Bloomington, IN, USA) successfully controlled the bleeding (Fig. 3). After the procedure, the patient recovered gradually.
Go to : 
Rupture of the lumbar artery is typically associated with trauma or surgery.[1] Although rare, lumbar artery rupture without associated trauma may be related to anticoagulation therapy.[2,3]
Retroperitoneal hematoma can present clinically as groin, flank, abdominal or back pain. In most cases, the onset is clinically marked by sudden violent pain. Management usually involves the correction of coagulopathy, fluid resus citation, blood transfusion, and other supportive measures. Angiography and arterial embolization may provide an efficacious therapy. Cases that are unresponsive to the aforementioned therapies are generally treated with surgery. In our patient, there was no abdominal trauma. The risk factors for bleeding included chronic kidney disease (CKD) and heparin use for CRRT.
Spontaneous bleeding in CKD patients is not uncommon; however, spontaneous lumbar artery rupture in CKD patients is quite rare. Halak et al.[4] reported the first case of spontaneous lumbar artery rupture in a hemodialysis patient in 2001. In Korea, Pyo et al.[5] reported spontaneous rupture of the lumbar artery and inferior epigastric artery in a hemodialysis patient. The origin of the bleeding tendency exhibited by CKD patients is multifactorial. Decreased platelet factor III activity, platelet aggregation and adhesiveness abnormalities, and impaired prothrombin consumption contribute to clotting defects, uremic thrombocytopenia and/or direct injury to the vascular intimal layer.[6,7] Abnormal bleeding times and coagulopathy may be reversed with desmopressin, cryoprecipitate, conjugated estrogen, or blood transfusion, as well as by the use of erythropoietin.[8]
CRRT is an extracorporeal blood purification therapy intended to substitute for impaired renal function over an extended period of time; it is administered 24 hours a day. [9] The use of CRRT in critically ill patients with acute renal failure, combined with cardiovascular instability, severe fluid overload, cerebral edema or hypercatabolism and high fluid requirements, is widely accepted. It can also be used in patients in refractory chronic edematous states or with pulmonary edema.[10]
One requirement of CRRT that has potential deleterious consequences is anticoagulation. While anticoagulation exposes patients to the risk of bleeding, the absence of it may result in clotting of the CRRT circuit. Bleeding during anticoagulation for CRRT is reported in 5-25% of patients,[11-13] although the incidence depends on the population and the criteria used to define bleeding. Optimal anticoagulation methods are easy to implement and monitor, have few side effects, and are cost effective.
Common treatment options include unfractionated heparin, regional citrate, no anticoagulation, and thrombin antagonists. Less commonly used modalities include heparin with protamine reversal, low-molecular-weight heparins, heparinoids, and platelet-inhibiting agents.
Unfractionated heparin (UFH) remains the most widely used anticoagulant for CRRT. The risk of hemorrhage is the main drawback to UFH. The reported incidence of bleeding ranges from 10-50%, with mortality due to bleeding as high as 15%.[14-16] It has been suggested that the risk of hemorrhage or clotting of the extracorporeal circuit is a function of aPTT rather than heparin dose.[14]
Regional citrate can be used for patients who are at high risk for bleeding. When citrate is used, metabolic monitoring is necessary and the composition of the CRRT fluids must be adjusted. Meta-analysis revealed longer circuit survival and less bleeding with the use of citrate compared with UFH.[17] However, citrate is now being replaced by other anticoagulants in Korea, because citrate anticoagulation is labor-intensive and calcium-free dialysate is not available in Korea.
Argatroban (Novastan®) is a direct thrombin inhibitor. It can be used in patients on renal replacement therapy (RRT) who develop heparin-induced thrombocytopenia. In one of the largest reported studies on RRT and argatroban, safety and efficacy were evaluated in 47 patients, including 14 on CRRT.[18] Major bleeding occurred in 3 of 50 treatment courses (6%). Argatroban is available in Korea, but clinical experience of its use in CRRT is limited.
Nafamostat mesilate (Futhan®), a synthetic serine protease inhibitor, is a prostacyclin analog. It has been suggested to be safer than regional or low-dose heparin. A few studies of nafamostat mesilate use in CRRT have been published; they reported less bleeding than seen with the use of UFH.[19] Nafamostat mesilate is now used widely in patients who are at high risk for bleeding.
As CRRT is increasingly used in the critical care/ICU setting, the need for anticoagulation presents greater challenges because many critically ill patients with sepsis and inflammation are already at higher risk of bleeding as well as clotting.
In conclusion, we suggest that the possibility of spontaneous retroperitoneal bleeding should be considered in CRRT patients with sudden decreases in hemoglobin. Anticoagulant selection for CRRT should be determined based on patient characteristics, local expertise, nursing comfort and ease of monitoring.
Go to : 
References
1. Sclafani SJ, Florence LO, Phillips TF, Scalea TM, Glanz S, Goldstein AS, et al. Lumbar arterial injury: radiologic diagnosis and management. Radiology. 1987; 165:709–14.

2. Fortina M, Carta S, Del Vecchio EO, Crainz E, Urgelli S, Ferrata P. Retroperitoneal hematoma due to spontaneous lumbar artery rupture during fondaparinux treatment. Case report and review of the literature. Acta Biomed. 2007; 78:46–50.
3. Pathi R, Voyvodic F, Thompson WR. Spontaneous extraperitoneal haemorrhage: computed tomography diagnosis and treatment by selective arterial embolization. Australas Radiol. 2004; 48:123–8.

4. Halak M, Kligman M, Loberman Z, Eyal E, Karmeli R. Spontaneous ruptured lumbar artery in a chronic renal failure patient. Eur J Vasc Endovasc Surg. 2001; 21:569–71.

5. Pyo SH, Seo JW, Park JH, Kim KP, Kim SH, Chang JW, et al. Spontaneous rupture of lumbar artery and inferior epigastric artery in a hemodialysis patient. Korean J Nephrol. 2004; 23:992–6.
6. Anderson S, Brenner BM. Progressive renal disease: A disorder of adaptation. Q J Med. 1989; 70:185–9.
7. Sohal AS, Gangji AS, Crowther MA, Treleaven D. Uremic bleeding: pathophysiology and clinical risk factors. Thromb Res. 2006; 118:417–22.

8. Janssen MJ, van der Meulen J. The bleeding risk in chronic haemodialysis: preventive strategies in high-risk patients. Neth J Med. 1996; 48:198–207.

9. Bellomo R, Ronco C, Mehta RL. Nomenclature for continuous renal replacement therapies. Am J Kidney Dis. 1996; 28(Suppl 3):S2–7.

10. Silverstein ME, Ford CA, Lysaght MJ, Henderson LW. Treatment of severe fluid overload by ultrafiltration. N Engl J Med. 1974; 291:747–51.

11. Mehta RL. Anticoagulation strategies for continuous renal replacement therapies: what works? Am J Kidney Dis. 1996; 28(Suppl 3):S8–14.

12. Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet. 2000; 356:26–30.

13. Bouman CS, Oudemans-Van Straaten HM, Tijssen JG, Zandstra DF, Kesecioglu J. Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospective, randomized trial. Crit Care Med. 2002; 30:2205–11.

14. van de Wetering J, Westendorp RG, van der Hoeven JG, Stolk B, Feuth JD, Chang PC. Heparin use in continuous renal replacement procedures: the struggle between filter coagulation and patient hemorrhage. J Am Soc Nephrol. 1996; 7:145–50.

15. Davenport A, Will EJ, Davison AM. Comparison of the use of standard heparin and prostacyclin anticoagulation in spontaneous and pump-driven extracorporeal circuits in patients with combined acute renal and hepatic failure. Nephron. 1994; 66:431–7.

16. Martin PY, Chevrolet JC, Suter P, Favre H. Anticoagulation in patients treated by continuous venovenous hemofiltration: a retrospective study. Am J Kidney Dis. 1994; 24:806–12.

17. Oudemans-van Straaten HM, Wester JP, de Pont AC, Schetz MR. Anticoagulation strategies in continuous renal replacement therapy: can the choice be evidence based? Intensive Care Med. 2006; 32:188–202.

18. Reddy BV, Grossman EJ, Trevino SA, Hursting MJ, Murray PT. Argatroban anticoagulation in patients with heparin-induced thrombocytopenia requiring renal replacement therapy. Ann Pharmacother. 2005; 39:1601–5.

19. Ohtake Y, Hirasawa H, Sugai T, Oda S, Shiga H, Matsuda K, et al. Nafamostat mesilate as anticoagulant in continuous hemofiltration and continuous hemodiafiltration. Contrib Nephrol. 1991; 93:215–7.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download