Abstract
Colchicine poisoning is rare but can cause potentially life-threatening toxic complications such as hypovolemic shock, cardiovascular collapse and multiple organ failure. In this case report, we describe a case of a 20-year-old female who presented to the emergency department after suicidal ingestion of a toxic dose of colchicine. She developed thrombocytopenia, neutropenia and acute respiratory distress syndrome that required blood transfusion and administration of granulocyte colony stimulating factor for the prevention of infectious complications. With regard to the clinical manifestations of colchicine toxicity, we discussed suggested mechanisms.
Colchicine is a liposoluble alkaloid derived from the plants Colchicum autumnale and Gloriosa superba. It is effective in the treatment of acute flares of gouty arthritis and rheumatic diseases such as Behçet’s disease[1,2] and has also been used experimentally to treat various other diseases. However, its clinical use has been limited by its extremely narrow therapeutic window.
Overdose can lead to dysfunction in multiple organs and is associated with a high mortality rate that has been attributed to early collapse of the cardio-respiratory system or infectious/hemorrhagic complications of bone marrow suppression.[3] To date, definite therapy has not been established. Therefore, sharing knowledge concerning various clinical features and treatment experiences through case reports may be helpful for physicians in improving treatment outcomes. We report a case of a 20-year-old female who ingested a toxic dose of colchicine and developed severe neutropenia, thrombocytopenia, and acute respiratory distress syndrome (ARDS).
A 20-year-old female patient with a history of skin vasculitis and Behçet’s disease was transferred to our emergency department (ED) from a nearby hospital with hypotension following ingestion of approximately 63 0.6 mg colchicine tablets (approximately 37.8 mg; 0.74 mg/kg bodyweight) over 5 hours. She did not vomit or spit out the medication before presentation to the ED. During monitoring of vital signs at the nearby hospital, only normal saline was infused with intravenous H2-receptor antagonist administration.
On arrival to our ED 6 hours after ingestion, she was alert but had acute symptoms of epigastric pain and nausea. Her vital signs were as follows: blood pressure, 82/50 mmHg; pulse rate, 126 beats/min; respiration rate, 20 breaths/min; and body temperature, 36.5°C. Remarkable laboratory results were a leukocyte count of 51.6 × 103/µL, platelet count of 23.9 × 103/µL, and prothrombin time (PT) of 27.9 seconds with an international normalized ratio (INR) of 2.46.
Gastric lavage and activated charcoal were not considered due to late presentation to our ED. After central venous catheter insertion, dopamine (20 μg/kg/min) infusion was started and three units of fresh frozen plasma (FFP) were administered. After 1 hour, the blood pressure was increased to 90/60 mmHg, and she was transferred to the intensive care unit (ICU). On the first day after admission, thrombocytopenia (39.0 × 103/µL) and an increased INR (1.82) were still observed. The blood chemistry panel showed aspartate transaminase at 193 U/L, alanine transaminase at 129 U/L, amylase at 260 IU/L, alkaline phosphatase at 1,056 IU/L, and lactate dehydrogenase at 4,752 IU/L (Table 1). We applied a non-rebreather mask because decreased oxygen saturation (89% via a nasal cannula on O2 5 L/min) and tachypnea (23-35/min) were observed. On chest X-ray, bilateral lung field haziness was seen. On the second day, mechanical ventilation (MV) was applied because of aggravated tachypnea (> 40/min) and hypoxia (88% via a non-rebreather mask on O2 9 L/min). In addition, urine output (approximately 10-30 mL/hr.) was decreased and intravenous (IV) furosemide (lasix) 20 mg was administered three times on suspicion of ARDS or pulmonary edema. IV phenytoin (500 mg/day maintenance after 1,000 mg loading) and IV antibiotics (cefoperazone at 1 g/day and aztreonam at 3 g/day) were administered because seizure-like movement was seen for approximately 30 seconds and the patient developed fever over 39°C. On hospital day 3, lenograstim at 250 μg (neutrogin, 5 μg/kg/day) was administered subcutaneously due to a low absolute neutrophil count (ANC; 0.46 × 103/µL). After that, ANC increased gradually and additional lenograstim was not given (Fig. 1). However, FFP and platelets were transfused until platelet and PT INR levels did not decrease without transfusion (Fig. 1). Continuous furosemide (20 mg/hour) infusion was started to avoid fluid overload. On hospital day 5, furosemide infusion was stopped and dopamine was tapered concurrently to 10 μg/kg/min from 20 μg/kg/min. Adequate urine output (approximately 100-150 mL/hr.) without furosemide administration was observed from hospital day 7, and dopamine infusion was discontinued on hospital day 6. The mode of MV was changed from assist-control mechanical ventilation (ACMV) to synchronized intermittent mandatory ventilation with pressure support. However, oxygen saturation on pulse oximetry decreased below 90%. Thus, ACMV mode was used again, and the patient was extubated from the MV after 4 days of ventilator support (ACMV mode). After the sedative drug (midazolam, 3 mg/hr.) used during the MV was discontinued, seizure-like movements were not observed. An electroencephalogram obtained on hospital day 12 showed no epileptiform discharge, and phenytoin administration was stopped after a neurologic consultation. Echocardiography was performed on hospital day 15, and abnormal findings were not observed. The patient was transferred to a general ward on hospital day 17 and discharged from the hospital with alopecia on hospital day 23.
Acute toxic manifestations of colchicine poisoning can be divided broadly into four stages. Bone marrow suppression is a predominant finding in stage 3, which occurs on days 3 to 6 post-exposure and continues for 7 to 10 days.[3] Prior to bone marrow suppression, gastrointestinal symptoms with volume depletion and multi-organ dysfunction may develop in stages 1 and 2, which begin 2 to 8 hours and 24 hours after ingestion, respectively. In stage 4, which begins 7 days after ingestion, generalized alopecia and rebound leukocytosis can be observed.
The ingestion of a toxic dose of colchicine, which ranges from 0.5 to 0.8 mg/kg, results in myelosuppression with 10% mortality, and ingestion of a lethal dose (>0.8 mg/kg) can lead to 100% mortality by cardiogenic shock.[4] Our patient reportedly ingested a toxic dose of approximately 37.8 mg of colchicine (0.74 mg/kg) over 5 hours. Her clinical manifestations were consistent with those of previous case reports. In the ED, she had epigastric pain, nausea, and hypotension, which can develop in stage 1 of colchicine intoxication. In the ICU, hemodynamic instability continued for several days and she showed abnormal blood test results: elevated liver, pancreas, muscle, and cardiac enzymes. Hypoxia due to ARDS or pulmonary edema with decreased urine output was also observed. Based on these clinical manifestations, we presume that several organs were affected by ingestion of a toxic dose of colchicine. Bone marrow suppression was somewhat different from previous reports in terms of the time when neutrophil and platelet counts began to decrease. In the present case, decreases in white blood cell and platelet counts were observed from admission to the ICU.
Disruption of the microtubular network has been suggested as a primary mechanism of colchicine-associated toxicity. By binding to intracellular α- and β-tubulin, colchicine prevents the formation of microtubules[5,6] and leads to disruption of the microtubular network. Then, alterations in normal cellular functions such as endocytosis and exocytosis, cellular motility, and mitosis occur, and finally cell division in all cell lines is inhibited. Through this mechanism, multi-organ dysfunction syndrome can develop; in particular, organs that have a high turnover rate, such as bone marrow or skin, are more vulnerable to this toxic effect.[7]
The main treatment of colchicine intoxication is supportive. If the patient presents to a hospital within 1-2 hours of ingestion, the physician can perform gastric lavage and administer activated charcoal.[8] If a large amount of colchicine ingestion is suspected, multiple-dose activated charcoal can be considered because colchicine has enterohepatic recirculation.[9]
In the early stage of colchicine intoxication, fluid resuscitation is important because acute renal injury can develop due to severe hypovolemia and muscle injury. Coagulation disorder and neutropenia should be managed with blood product transfusions and granulocyte colony-stimulating factor (G-CSF) administration. There are a few reported cases in which G-CSF was used in the treatment of colchicine poisoning.[3,10-14] In these cases, G-CSF was suggested as supportive therapy for leukopenia because it shortened the duration of neutropenia and could help prevent the development of septicemia by accelerating the production of neutrophils within the bone marrow. Broad-spectrum antibiotics should also be considered if there are clinical signs suggestive of infection. In our patient, neutropenia recovered after a single dose of G-CSF. However, there are cases in which G-CSF was given for several days for neutropenia,[3,10] and patient outcome was poor despite G-CSF administration.[12-14]
In one case of colchicine poisoning, treatment with colchicine-specific Fab fragment antibodies was effective.[15] The efficacy was explained by the high affinity of colchicine-specific antibodies to colchicine, which immunoneutralized the effects of colchicine. However, these fragments are not available in clinical practice.
We assumed that clinical features in our patient were typical and, fortunately, less severe than expected considering the amount of colchicine ingested. Her young age and good response to treatment also seemed to contribute to her recovery without major complications.
In conclusion, colchicine poisoning can lead to life-threatening multi-organ damage, and there are no therapeutic methods to eliminate colchicine. Thus, aggressive supportive care should be performed from the time of presentation to prevent progression to multi-organ failure.
ACKNOWLEDGMENTS
This work was supported by 2015 clinical research grant from Pusan National University Hospital.
References
2. Lange U, Schumann C, Schmidt KL. Current aspects of colchicine therapy -- classical indications and new therapeutic uses. Eur J Med Res. 2001; 6:150–60.
3. Harris R, Marx G, Gillet M, Kark A, Arunanthy S. Colchicine-induced bone marrow suppression: treatment with granulocyte colony-stimulating factor. J Emerg Med. 2000; 18:435–40.

4. Cocco G, Chu DC, Pandolfi S. Colchicine in clinical medicine. A guide for internists. Eur J Intern Med. 2010; 21:503–8.

5. Andreu JM, Timasheff SN. Tubulin bound to colchicines forms polymers different from microtubules. Proc Natl Acad Sci U S A. 1982; 79:6753–6.
6. Luduena RF, Roach MC. Tubulin sulfhydryl groups as probes and targets for antimitotic and antimicrotubule agents. Pharmacol Ther. 1991; 49:133–52.

8. Murray SS, Kramlinger KG, McMichan JC, Mohr DN. Acute toxicity after excessive ingestion of colchicine. Mayo Clin Proc. 1983; 58:528–32.
9. Borron SW, Scherrmann JM, Baud FJ. Markedly altered colchicines kinetics in a fatal intoxication: examination of contributing factors. Hum Exp Toxicol. 1996; 15:885–90.
10. Folpini A, Furfori P. Colchicine toxicity--clinical features and treatment. Massive overdose case report. J Toxicol Clin Toxicol. 1995; 33:71–7.
11. Critchley JA, Critchley LA, Yeung EA, Young RP, Young RJ, Chan TY, et al. Granulocyte-colony stimulating factor in the treatment of colchicines poisoning. Hum Exp Toxicol. 1997; 16:229–32.
12. Lin PF, Cheng CY, Fang CL, Sue YM. Colchicine poisoning: an unusual cause of diarrhea with multiorgan failure. J Exp Clin Med. 2013; 5:235–6.

13. Milne ST, Meek PD. Fatal colchicine overdose: report of a case and review of the literature. Am J Emerg Med. 1988; 16:603–8.

14. Little A, Tung D, Truong C, Lapinsky S, Burry L. Colchicine overdose with coingestion of nonsteroidal anti-inflammatory drugs. CJEM. 2014; 16:252–6.

15. Baud FJ, Sabouraud A, Vicaut E, Taboulet P, Lang J, Bismuth C, et al. Brief report: treatment of severe colchicine overdose with colchicine-specific Fab fragments. N Engl J Med. 1995; 332:642–5.
Fig. 1.
Serial changes in white blood cell count (A), platelet count and the volume of transfused platelet concentration (B), international normalized ratio and volume of transfused fresh frozen plasma (C), and aspartate transaminase and alanine transaminase (D) over time after presentation to the emergency department. Arrow indicates the time of administration of granulocyte colony stimulating factor (G-CSF). ED: emergency department; WBC: white blood cell; ANC: absolute neutrophil count; PLT: platelet; PT: prothrombin time; INR: international normalized ratio; FFP: fresh frozen plasma; AST: aspartate transaminase; ALT: alanine transaminase.
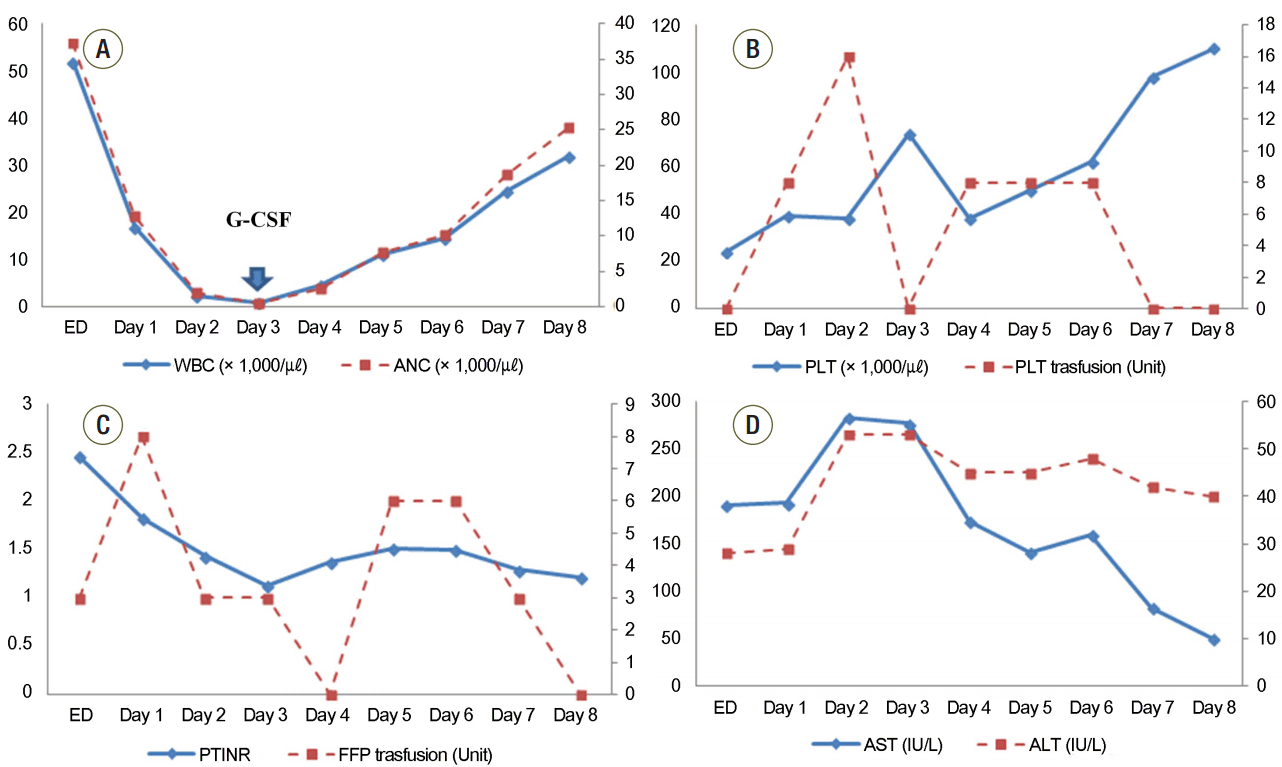
Table 1.
Laboratory findings in emergency department and intensive care unit
ED: emergency department; WBC: white blood cell; ANC: absolute neutrophil count; PLT: platelet; AST: aspartate aminotransferase; ALT: alanine aminotransferase; PTT: partial thromboplastin time; PT: prothrombin time; INR: international normalized ratio; BUN: blood urea nitrogen; CK-MB: creatine kinase-MB; ALP: alkaline phosphatase; LDH: lactate dehydrogenase; CRP: c-reactive protein.




 ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download