Abstract
Severe acute respiratory distress syndrome (ARDS) is a life-threatening disease with a high mortality rate. Although many therapeutic trials have been performed for improving the mortality of severe ARDS, limited strategies have demonstrated better outcomes. Recently, advanced rescue therapies such as extracorporeal membrane oxygenation (ECMO) made it possible to consider lung transplantation (LTPL) in patients with ARDS, but data is insufficient. We report a 62-year-old man who underwent LTPL due to ARDS with no underlying lung disease. He was admitted to the hospital due to influenza A pneumonia-induced ARDS. Although he was supported by ECMO, he progressively deteriorated. We judged that his lungs were irreversibly damaged and decided he needed to undergo LTPL. Finally, bilateral sequential double-lung transplantation was successfully performed. He has since been alive for three years. Conclusively, we demonstrate that LTPL can be a therapeutic option in patients with severe ARDS refractory to conventional therapies.
Severe acute respiratory distress syndrome (ARDS) is a life-threatening disease with a high mortality rate.[1] Many therapeutic trials have been attempted to improve this situation with little success. Since the pandemic H1N1 period in 2009, extracorporeal oxygenation (ECMO) therapy has become one of the common rescue treatments for severe ARDS.[2] With the use of advanced rescue therapies, such as ECMO support, lung transplantation (LTPL) has been attempted an alternative therapeutic approach for severe ARDS. LTPL could also represent a potential approach to consider in cases of refractory hypoxemia.[1] There are limited data on the use of LTPL as a therapeutic option in cases of severe ARDS but we here report a case of influenza pneumonia-induced ARDS that was successfully treated in this way.
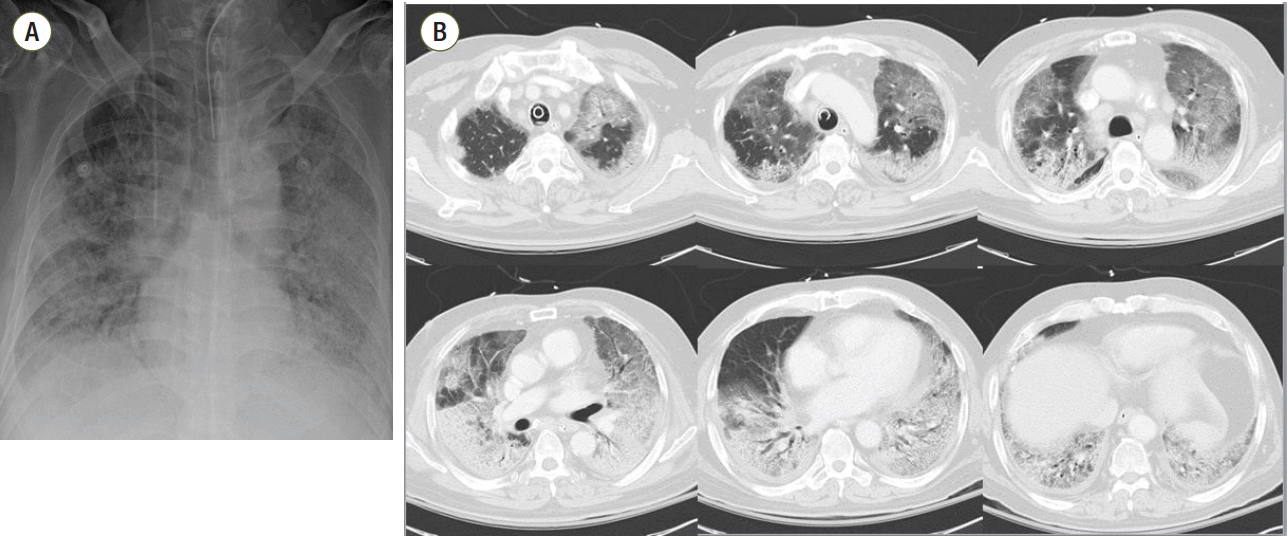
A 62-year-old man had been admitted to our hospital due to the onset of dyspnea with cough and sputum 3 days earlier. He had diabetes mellitus and hypertension that was being managed with medication. His initial vital signs were: blood pressure, 115/70 mmHg; heart rate, 95 beats/min; respiratory rate, 26 breaths/min; and body temperature, 38.4°C. Chest radiography and computed tomography scans revealed dominant bilateral pulmonary infiltration with diffuse ground glass opacity (Fig. 1). There was no evidence of left ventricular dysfunction or increased left atrial pressure. ARDS was suspected because of pneumonia. After admission, his hypoxemia worsened and he underwent invasive mechanical ventilation. Despite the application of positive end expiratory pressure (PEEP) of 18 cmH2O with FiO2 of 1.0, his PaO2/FiO2 ratio (P/F ratio) was only 62 mmHg, which was suggestive of severe ARDS according to the Berlin definition. Bronchoalveolar lavage was carried out on hospital day (HD) 1 and yielded positive influenza A virus PCR results with no other pathogen.
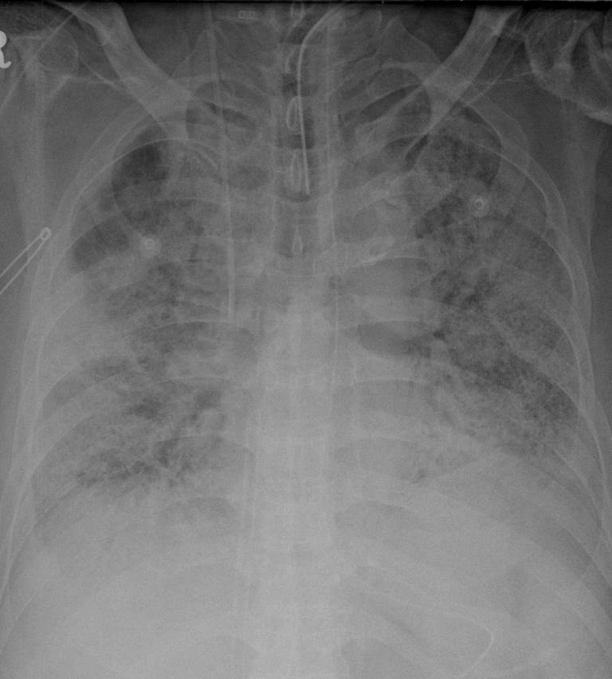
He was diagnosed with ARDS because of the influenza A pneumonia and was treated with antiviral agents. We administered oseltamivir for the first 2 days followed by intravenous peramivir for the next 12 days. However, his fever continued and his oxygen requirement increased. Methicillin-resistant Staphylococcus aureus (MRSA) was isolated from his tracheal aspirates, and he was subsequently assessed as having superimposed MRSA pneumonia. His P/F ratio consistently deteriorated to less than 80 mmHg with a PEEP of 16 cmH2O. It was difficult to put him in a prone position because of a tracheostomy which has been performed earlier. Inhaled nitric oxide was applied at HD 11 to treat his persistent hypoxemia. However, his lung condition worsened and so he underwent a venous-venous ECMO at HD 17 (Fig. 2). His follow-up bronchoalveolar lavage did not show additional pathogens, and he was then started on a regimen of steroid therapy with 2 mg/kg/day methylprednisolone. Subsequently, his lung showed gradual improvement and we then attempted to wean him from the ECMO. He tolerated a full ECMO weaning trial with a mild increase in FiO2 with a mechanical ventilator from 0.4 to 0.5. His ECMO was removed at HD 24. However, a few days later, his P/F ratio again deteriorated, even though he had continued on steroid therapy. Finally, he again required ECMO support at HD 30. We believed at that time that his lung condition would not be reversible because of his clinical course, and decided to conduct LTPL as a last therapeutic option. He did not have any other signs of organ failure except for respiratory failure. He showed a persistent low P/F ratio of <100 mmHg and exhibited fibrotic changes on chest radiography. It appeared to be difficult to wean him from the ECMO.
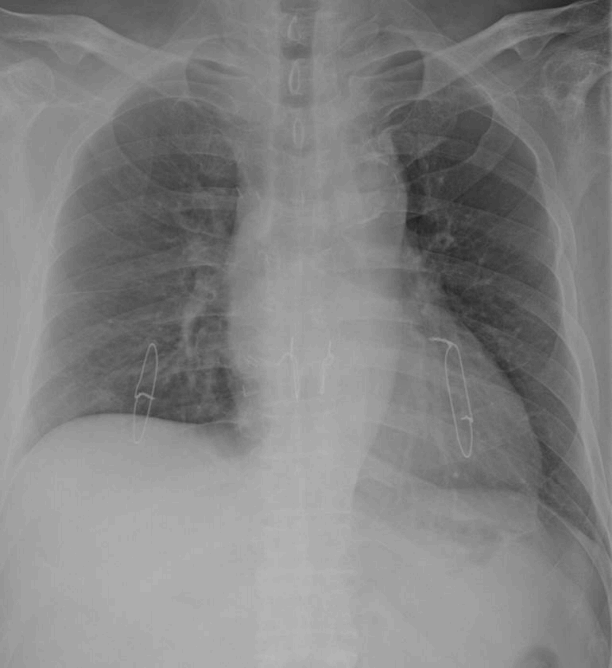
After discussion with the patient and his family members about his anticipated clinical course and expected problems after LTPL, the LTPL procedure was agreed to. He was listed on the Korean Network for Organ Sharing at HD 35. During the waiting period for an appropriate donor, he developed hypotension as a result of right ventricular (RV) dysfunction with pulmonary hypertension. His condition deteriorated and the venous-venous ECMO was switched to venous-venous-arterial (VV-A) for his persistent hypotension at HD 43. However, he then developed two-circulation syndrome, and thereafter his oxygenation could not be recovered above an SaO2 of 85%. The LTPL procedure then became critical for the patient’s survival. Fortunately, an appropriate donor was identified two days later and at HD 45, he underwent a sequential double-lung transplantation. His postoperative course was uneventful. His RV dysfunction recovered and then he was weaned from mechanical ventilation at HD 58 (postoperative day [POD] 18). He was transferred to the general ward at HD 62 (POD 22). At the time of writing, he remains alive at 3 years and 1 month post-LTPL (Fig. 3).
We here describe a 62 year old male patient who underwent LTPL as a final therapeutic option for ARDS induced by influenza pneumonia that was refractory to conventional therapies. This case illustrates that in cases with no underlying lung disease or other organ failure, LTPL is a possible final therapeutic option for refractory ARDS, even in older patients. To date, some cases of severe ARDS who ultimately underwent LTPL have been reported.[3-12] Among these reported cases however, pneumonia-induced ARDS patients who were treated with LTPL are rare.[10] In addition, the cases of this type that have been reported previously were all teenagers whereas our current case report is distinct as our patient was a much older man.
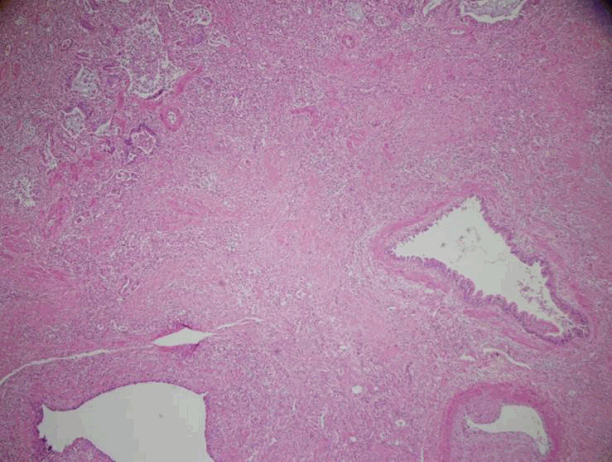
LTPL has been typically conducted in patients with irreversible end-stage lung disease, such as interstitial pulmonary fibrosis or chronic obstructive pulmonary disease.[13] The recent applications of advanced life support, such as ECMO, have made it possible for patients with acute respiratory failure, such as acute deterioration from chronic lung disease or severe ARDS, to undergo LTPL.[1] However, the indications for LTPL among such patients remain controversial. Regarding ARDS, many arguments for indications for LTPL have been put forward to date [14,15] and the main issue has been the reversibility of ARDS. Our current ARDS case also raised the question of reversibility but the LTPL could not have been delayed because his hypoxemia did not improve, even after VV-A ECMO was applied. His RV failure was not expected to have recovered if his lung had not improved. One month has elapsed at that time after the onset of pneumonia-induced ARDS. We judged that the ARDS-induced lung fibrosis, and expected ventilator-induced lung injury following continued mechanical ventilation over 2 weeks, would continue to progress and that the patient would soon die without this surgery. In our view therefore, his clinical status was irreversible. After LTPL, the pathology of his explanted lung exhibited end stage fibrosis and multifocal honeycomb changes that were consistent with fibrotic phase diffuse alveolar damage (Fig. 4). These findings supported our clinical judgment.
Our current case is also an example of the use of ECMO therapy as a bridge to LTPL. Soon after the introduction of extracorporeal life support for respiratory failure, ECMO was not recommended as a therapy for ARDS because of its high associated mortality.[16] However, recent advances in ECMO technology have enabled the possibility of achieving a survival benefit with this intervention in patients with severe ARDS.[2,17] Despite the many controversies surrounding it, the recent CESAR trial[17] did indicate that ECMO might improve the outcomes of patients with severe refractory hypoxemia if they did not respond to other conventional rescue therapies and could be transferred to a center that could provide this treatment.[1] Recently, ECMO therapy has been attempted as a bridge to LTPL at many centers.[12] Because the use of ECMO itself cannot guarantee recovery from the underlying lung disease, it should not be applied in any patient with a condition that is expected to be irreversible. LTPL is an effective life-saving treatment for patients with irreversible end-stage lung disease, so the use of ECMO as a bridge to LTPL could be considered acceptable in such cases. In our present case, the first ECMO therapy two weeks after intensive care unit admission was administered on the expectation that he could improve within a certain period of time. However, when we elected for a second application of ECMO because of the subsequent rapid deterioration that followed weaning from his first ECMO therapy, our decision was made under the premise that he might need LTPL as the ultimate treatment. Indeed, he had a relative contraindication for ECMO therapy i.e. prolonged mechanical ventilation for more than 7 days. However, in accordance with current international guidelines,[18] he had no organ dysfunction other than respiratory failure and no absolute contraindication, such as a precluding anticoagulation therapy. Thus, we elected for him to receive a second round of ECMO therapy.
To date, the use of LTPL to treat refractory acute respiratory failure without underlying lung disease has mostly been reported in patients with paraquat poisoning,[3,4] posttraumatic ARDS,[6] inhalation injury,[9] acute interstitial pneumonia,[11,12] sepsis-induced ARDS,[5] and pneumonia-induced ARDS.[10] Some instances of this intervention have been reported in patients with post-ARDS fibrosis undergoing long-term mechanical ventilation.[19] However, most of these patients were young and had no underlying illnesses. In practice, it is difficult for physicians to wait and see how the condition of a patient will develop, especially when they are young with no previous lung diseases or serious comorbidities. For this reason, LTPL has been attempted as the final option in such cases. The long-term outcome of ARDS patients who undergo LTPL remains unknown. Based on a recent literature search that we conducted, a total of 17 ARDS patients that underwent LTPL have been reported. Among these cases, 14 patients whose survival times were reported showed two different survival patterns—early mortality within 3 months (4 patients) and long-term survival for 1to 5 years (10 patients). Our patient belongs to the latter group with a survival time of more than 3 years. Based on a recent report, patients who received LTPL supported with ECMO had a higher rate of perioperative mortality compared with those without ECMO, but an acceptable mid-term survival outcome has been shown in selected patients. [20] There was no significant difference reported in the survival rates at 1-year (74% versus 78%) and 3-years (65% versus 62%) after LTPL between patients with and without ECMO support.[20] That study did not include ARDS patients, but most case reports of ARDS patients treated with LTPL describe using supporting ECMO. Therefore, our current case shows similarities with previously reported ARDS patients. Taken together, previous data and our current case suggest that LTPL could represent an additional therapeutic option in properly selected patients with refractory severe ARDS.
In conclusion, LTPL bridged with ECMO was successfully carried out to achieve long-term survival in an older patient with severe ARDS that was refractory to conventional therapies. We found that if a patient suffered from life-threatening ARDS that is refractory to other rescue therapies, LTPL can represent a therapeutic option. However, the careful selection of patients without concurrent organ dysfunction or serious comorbidities should be ensured to achieve success with this approach.
References
1. Pipeling MR, Fan E. Therapies for refractory hypoxemia in acute respiratory distress syndrome. JAMA. 2010; 304:2521–7.

2. Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investingators, Davies A, Jones D, Bailey M, Beca J, Bellomo R, et al. Extracorporeal membrane oxygenation for 2009 Influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009; 302:1888–95.

3. Sequential bilateral lung transplantation for paraquat poisoning. A case report. The Toronto Lung Transplant group. J Thorac Cardiovasc Surg. 1985; 89:734–42.
4. Egan TM, Duffin J, Glynn MF, Todd TR, DeMajo W, Murphy E, et al. Ten-year experience with extracorporeal membrane oxygenation for severe respiratory failure. Chest. 1988; 94:681–7.

5. Tsang V, Evans TW, Morgan C, Yacoub M. Heart-lung transplantation for adult respiratory distress syndrome. Crit Care Med. 1991; 19:286–7.

6. Demertzis S, Haverich A, Ziemer G, Nerlich M, Müller KM, Wagner TO, et al. Successful lung transplantation for posttraumatic adult respiratory distress syndrome after extracorporeal membrane oxygenation support. J Heart Lung Transplant. 1992; 11:1005–7.
7. Brichon PY, Barnoud D, Pison C, Perez I, Guignier M. Double lung transplantation for adult respiratory distress syndrome after recombinant interleukin 2. Chest. 1993; 104:609–10.

8. Licker M, Schweizer A, Hohn L, Morel DR, Spiliopoulos A. Single lung transplantation for adult respiratory distress syndrome after paraquat poisoning. Thorax. 1998; 53:620–1.

9. Barrio J, Sánchez C, Vicente R, Ramos F, Montero R, Morales P, et al. Successful sequential double-lung transplantation for adult respiratory distress syndrome after long-term mechanical ventilation. Eur J Anaesthesiol. 2004; 21:326–7.

10. Jackson A, Cropper J, Pye R, Junius F, Malouf M, Glanville A. Use of extracorporeal membrane oxygenation as a bridge to primary lung transplant: 3 consecutive, successful cases and a review of the literature. J Heart Lung Transplant. 2008; 27:348–52.

11. Nosotti M, Rosso L, Palleschi A, Lissoni A, Crotti S, Marenghi C, et al. Bridge to lung transplantation by venovenous extracorporeal membrane oxygenation: a lesson learned on the first four cases. Transplant Proc. 2010; 42:1259–61.

12. Hämmäinen P, Schersten H, Lemström K, Riise GC, Kukkonen S, Swärd K, et al. Usefulness of extracorporeal membrane oxygenation as a bridge to lung transplantation: a descriptive study. J Heart Lung Transplant. 2011; 30:103–7.

13. Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, et al. The registry of the International Society for Heart and Lung Transplantation: twentysixth official adult lung and heart-lung transplantation report-2009. J Heart Lung Transplant. 2009; 28:1031–49.

14. Egan TM. When is lung transplantation appropriate? J Heart Lung Transplant. 1992; 11:1008.
15. Navarro R. Lung transplantation in adult respiratory distress syndrome. J Heart Lung Transplant. 1993; 12:880.
16. Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979; 242:2193–6.

17. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009; 374:1351–63.

18. Orens JB, Estenne M, Arcasoy S, Conte JV, Corris P, Egan JJ, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006; 25:745–55.





 ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download