Abstract
Conventional medical therapies have not been very successful in treating adults with refractory septic shock. The effects of direct hemoperfusion using polymyxin B and veno-arterial extracorporeal membrane oxygenation (ECMO) for refractory septic shock remain uncertain. A 66-year-old man was admitted to the emergency department and suffered from sepsis-induced hemodynamic collapse. For hemodynamic improvement, we performed direct hemoperfusion using polymyxin B. Computed tomography scan of this patient revealed emphysematous pyelonephritis (EPN), for which he underwent emergent nephrectomy with veno-arterial ECMO support. To the best of our knowledge, this is the first report of successful treatment of EPN with refractory septic shock using polymyxin B hemoperfusion and nephrectomy under the support of ECMO.
It is difficult to treat adults with septic shock, which is refractory to adequate intravascular volume and infusion of very high-dose catecholamines. Endotoxin plays an important role in the pathogenesis of septic shock. Polymyxin B hemoperfusion aims to remove circulating endotoxin by adsorption, theoretically preventing the progression of the biological cascade of sepsis. In a systematic review, we found that when direct hemoperfusion with the polymyxin B (PMX-DHP) was carried out, there were significant improvements in the hemodynamics, pulmonary oxygenation, and mortality of patients with septic shock.[1] However, we do not have sufficient evidence to prove that PMX-DHP was applied while conducting rescue therapy on patients with refractory septic shock. To treat neonates and children with refractory septic shock, the American College of Critical Care Medicine has recommended extracorporeal membrane oxygenation (ECMO) as a viable therapy.[2] Several studies have reported the use of ECMO in the treatment of adult patients with refractory septic shock. However, its efficacy remains controversial.[3-5] In this report, we describe how a patient with emphysematous pyelonephritis (EPN) and refractory septic shock was successfully treated with PMX-DHP and nephrectomy under the support of ECMO.
A 66-year-old man was admitted to our emergency department as he complained of severe fever and chills for the past two days. He was a diabetic and hypertensive patient who received regular medication.
At presentation, his systolic blood pressure was 91 mmHg, while his diastolic blood pressure was 61 mmHg. His pulse rate was 124 beats per minute, while his respiration rate was 18 times per minute. The body temperature of this patient was 37.1°C. Physical examination indicated right costovertebral angle tenderness. The laboratory test results of this patient were as follows: hemoglobin: 11.5 g/dL, total leukocyte count: 20,050 cells/µL (segmented neutrophils: 79.5%), platelet count: 245,000 cells/µL, blood urea nitrogen: 25.1 mg/dL, creatinine: 1.52 mg/dL, total bilirubin: 0.8 mg/dL, C-reactive protein: 16.22 mg/dL, procalcitonin: 0.57ng/mL, and lactate: 2.58 mmol/L. His urine microscopy revealed bacteriuria and pyuria. Compared to the previous chest radiograph of this patient, there was no significant interval change.
Early goal-directed therapy and piperacillin/tazobactam was initiated. But, the blood pressure of this patient dropped to 74/42 mmHg despite administering sufficient amount of fluid. Even though high dose of vasopressor, the patient showed confused mentality and pulse was impalpable. So, cardiopulmonary resuscitation (CPR) was performed. After performing CPR for 5 minutes, spontaneous circulation was restored. Severe metabolic and lactic acidosis was assumed to be the cause of cardiac arrest.
This patient had developed sepsis of intra-abdominal origin with unstable hemodynamics. So, we decided to treat him with PMX-DHP. 3 hours of PMX-DHP was performed using a blood flow rate of 120 mL/min and nafamostat as the anticoagulant. Immediately before conducting hemoperfusion, the infusion rate of norepinephrine was increased to 1.5 µg/kg/min and the infusion rate of vasopressin was 0.03 ug/kg/min. But after initiating the PMX-DHP therapy, we tapered the infusion levels of vasopressin and norepinephrine. The second session of PMX-DHP was performed on the next day. Twenty-four hours after the initiation of PMXDHP therapy, we tapered the infusion level of norepinephrine to 0.3 µg/kg/min.
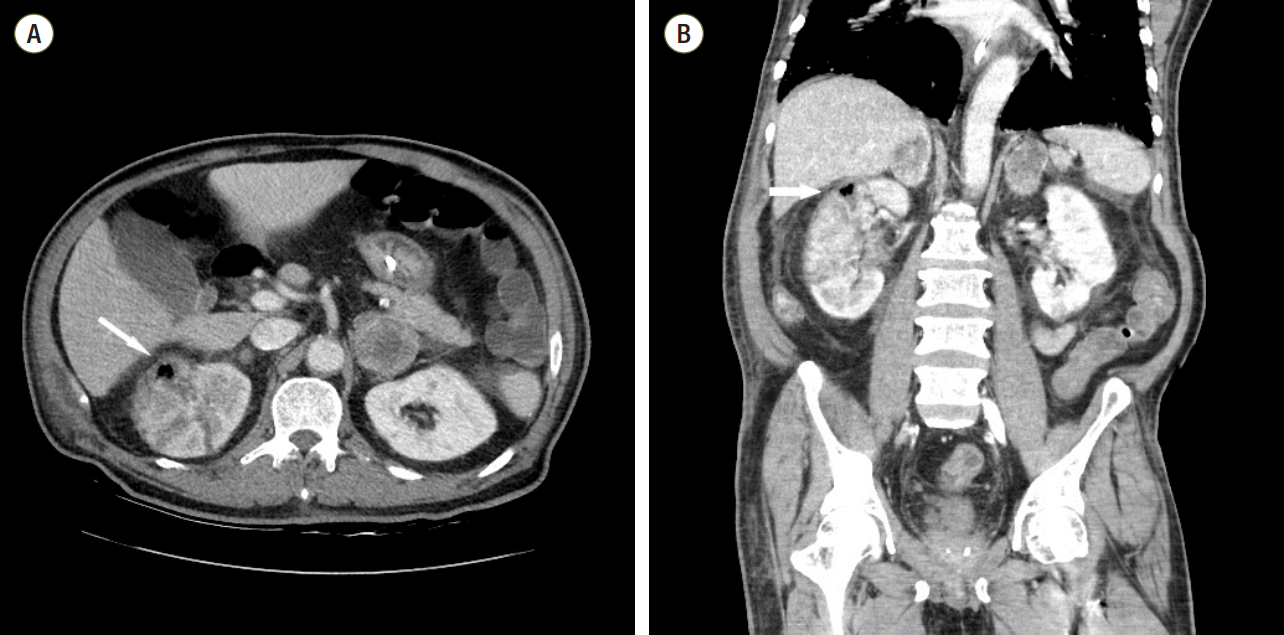
Despite subjecting the patient to PMX-DHP therapy, he continued to have high grade fever and still needed vasopressor support. Computed tomography (CT) scan was conducted to rule out the development of uncontrolled infection. CT scan of abdomen showed swelling and multifocal perfusion defect in both kidneys. It also detected the gas in the right renal parenchyma (Fig. 1). After examining the CT scan, we decided to perform emergency nephrectomy. But, the pulse rate of this patient had increased to 160 beats/min, and his cardiac markers were also consistently elevated. Bedside echocardiography detected severe global hypokinesia of the left ventricle. The ejection fraction of left ventricle was just 20%. And, more vasopressor was required to maintain blood pressure. In addition, the arterial oxygen saturation was in the range of 85-90%, though fraction of inspired oxygen was 1.0. Despite being subjected to vasopressor and ventilator support, the hemodynamic and ventilator parameters of the patient deteriorated further. So, they were considered to be incompatible with the operation. Under these circumstances, we decided to apply a veno-arterial ECMO. ECMO was promptly established by percutaneous technique. We used RotaFlow® (Maquet Inc., Hirrlingen, Germany), and the ECMO flow was maintained at 3.7 L/min.
Under ECMO support, we performed open simple nephrectomy on the patient. After the operation, the patient showed remarkable improvement in his hemodynamic parameters. But, the patient also incurred neurologic deficit as he developed left sided weakness and semicomatous mentality. The patient’s brain magnetic resonance imaging revealed an acute right fronto-parietal cerebral infarction. The proximal right internal carotid artery was almost totally occluded as it underwent severe stenosis.
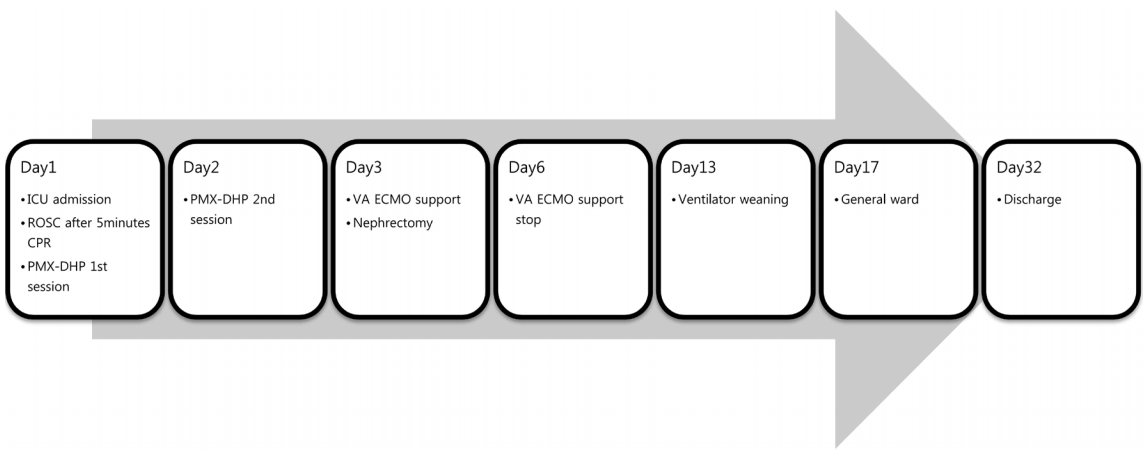
The patient was treated with ECMO for three more days. ECMO support was successfully discontinued on the sixth day of his stay in the hospital. Thereafter, he was on ventilator support for one more week because of mentality. Fourteen days after the operation, he was transferred to the general ward and his mental state improved enough to decipher instructions. And the follow-up echocardiography showed normal left ventricular systolic function. He was discharged on the 32nd day of his hospital stay. The brief course of the patient is shown in Fig. 2.
The patient experienced EPN and refractory septic shock. Septic shock of intra-abdominal origin is usually caused by either gram-negative bacteria or mixed pathogens. So, it is likely to be associated with high levels of endotoxin. The PMX-DHP column shows binds circulating endotoxin with high affinity, thereby decreasing the circulating endotoxin levels by up to 90% after two sessions of treatments. Clinical research studies have reported that PMX-DHP therapy results in improvement of hemodynamics and reductions in mortality.[1] More recently, the randomized controlled trial of surgical patients, with septic shock and severe sepsis, demonstrated statistically significant improvements in the hemodynamics.[6] This result supports that PMX-DHP therapy may be effective in patients, after controlling the infection source. However, in our case, the patient developed uncontrolled infection, and this is why his condition deteriorated even after being subjected to PMX-DHP therapy.
Severe cardiac dysfunction is reportedly observed in 17% of cases diagnosed with septic multiple organ dysfunction syndrome.[7] ECMO is expected to be effective in neonates and children who develop septic cardiomyopathy. These children undergo cardiogenic refractory shock despite being treated with conventional modes of treatment. In this situation, the ECMO could provide extra time for recovery of the failing heart and support the perfusion of major organs until cardiac function spontaneously recovers and infection control can be achieved using antibiotics or interventions. However, the efficacy of mechanical circulatory assistance remains controversial in adults with refractory septic shock. The chances of survival and hospital discharge was 71% (10/14) in a study conducted by Brechot et al.[3] but only 15% (8/52) in a study conducted by Huang et al.[4] According to a recently released report of our hospital, the chances of survival and hospital discharge was 22% (7/32) despite providing such patients with ECMO support.[5] Recent studies[4,5] have reported favorable outcomes of ECMO support in patients with severe myocardial injury. But, unfavorable outcomes of ECMO support have also been reported in patients with age over 60 years and in patients who suffered cardiac arrest due to septic shock.[4,5] Central ECMO might be associated with increased survival in the pediatric population. The theoretical benefits of central cannulation include safely achieving higher ECMO flow rates and potentially reversing shock more quickly than peripheral cannulation.[8] So, some expert recommend the use of central ECMO or other modifications.[4] But, in adult septic shock, it has not been proved to be a valid approach.
PMX-DHP and ECMO can be considered as treatment options in patients with refractory septic shock. However, they may not be beneficial to all cases of refractory septic shock. Moreover, these treatment methods could also be very expensive and consume lot of resources. For instance, it cost about ten million won to do PMX-DHP therapy, and it cost about eight million won to cannulate and maintain the ECMO circuit for this patient. Therefore, these treatment options must be applied only on suitable cases of refractory septic shock. Research of PMX-DHP is in progress and we need more experience about ECMO for refractory septic shock. The specific suggestion of PMX-DHP or ECMO for refractory septic shock is over the scope of this case report. In this case report, we have described the positive outcome of using ECMO support on a patient who developed EPN and refractory cardiovascular dysfunction due to septic shock.
References
1. Cruz DN, Perazella MA, Bellomo R, de Cal M, Polanco N, Corradi V, et al. Effectiveness of polymyxin B-immobilized fiber column in sepsis: a systematic review. Crit Care. 2007; 11:R47.

2. Brierley J, Carcillo JA, Choong K, Cornell T, Decaen A, Deymann A, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009; 37:666–88.
3. Bréchot N, Luyt CE, Schmidt M, Leprince P, Trouillet JL, Léger P, et al. Venoarterial extracorporeal membrane oxygenation support for refractory cardiovascular dysfunction during severe bacterial septic shock. Crit Care Med. 2013; 41:1616–26.

4. Huang CT, Tsai YJ, Tsai PR, Ko WJ. Extracorporeal membrane oxygenation resuscitation in adult patients with refractory septic shock. J Thorac Cardiovasc Surg. 2013; 146:1041–6.

5. Park TK, Yang JH, Jeon K, Choi SH, Choi JH, Gwon HC, et al. Extracorporeal membrane oxygenation for refractory septic shock in adults. Eur J Cardiothorac Surg. 2015; 47:e68–74.

6. Cruz DN, Antonelli M, Fumagalli R, Foltran F, Brienza N, Donati A, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009; 301:2445–52.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download