Abstract
Background and Purpose
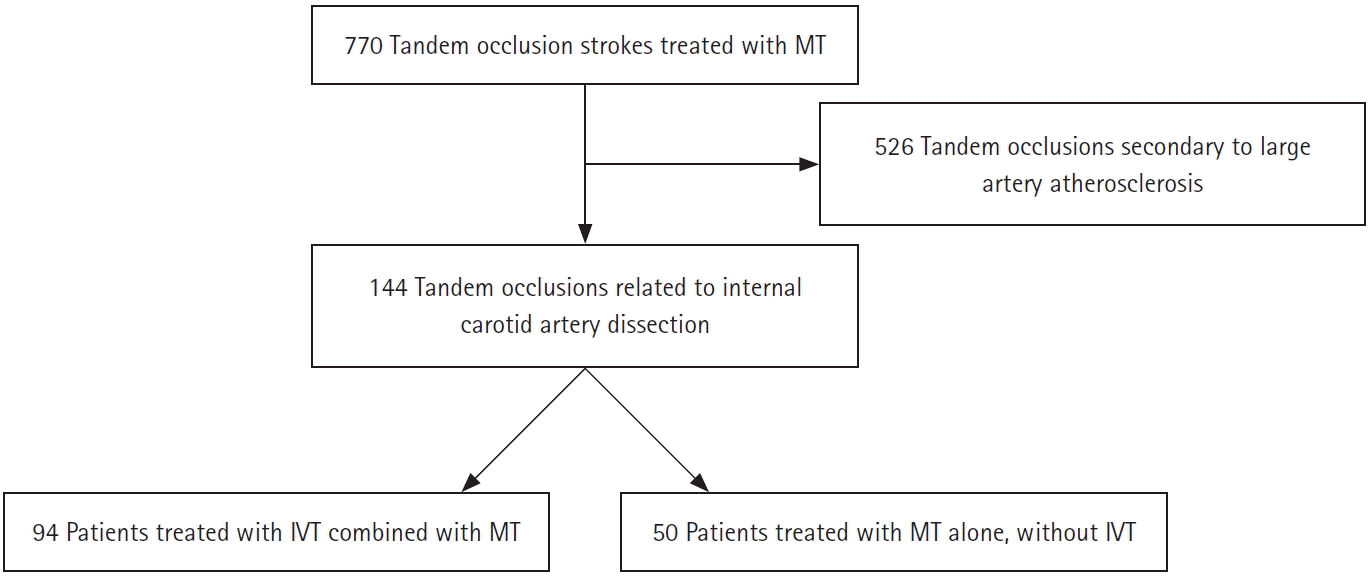
Methods
Results
Supplementary materials
Supplementary data 1
Notes
ETIS investigators
Michel Piotin, Raphaël Blanc, Simon Escalard, Jean-Philippe Desilles, Benjamin Maïer, Gabriele Ciccio, Stanislas Smajda, Mikael Mazighi, François Delvoye, Robert Fahed, Mikael Obadia, Candice Sabben, Ovide Corabianu, Thomas de Broucker, Didier Smadja, Sonia Alamowitch, Olivier Ille, Eric Manchon, PierreYves Garcia, Guillaume Taylor, Malek Ben Maacha.
Frédéric Bourdain, Jean-Pierre Decroix, Adrien Wang, Serge Evrard, Maya.Tchikviladze, Bertrand Lapergue, Oguzhan Coskun, Arturo Consoli, Federico Di Maria, Georges Rodesh, Morgan Leguen, Marie Tisserand, Fernando Pico, Haja Rakotoharinandrasana, Philippe Tassan, Roxanna Poll.
Gaultier Marnat, Florent Gariel, Xavier Barreau, Jérôme Berge, Patrice Menegon, Igor Sibon, Ludovic Lucas, Stéphane Olindo, Pauline Renou, Sharmila Sagnier, Mathilde Poli, Sabrina Debruxelles, François Rouanet, Thomas Tourdias, Jean-Sebastien Liegey, Isabelle Quintana, Tristan Kerdraon.
Romain Bourcier, Lili Detraz, Benjamin Daumas-Duport, Pierre-Louis Alexandre, Monica Roy, Cédric Lenoble, Vincent L’allinec, Jean-Baptiste Girot, Hubert Desal.
Benjamin Gory, Fatiha Bechiri, Sarah Guy, Serge Bracard, René Anxionnat, Marc Braun, Anne-Laure Derelle, Romain Tonnelet, Liang Liao, François Zhu, Emmanuelle Schmitt, Sophie Planel, Sébastien Richard, Lisa Humbertjean, Gioia Mione, Jean-Christophe Lacour, Gabriela Hossu, Marine Beaumont, Mitchelle Bailang, Gérard Audibert, Marie Reitter, Agnès Masson, Lionel Alb, Adriana Tabarna, Marcela Voicu, Iona Podar, Madalina Brezeanu.
Vincent Costalat, Cyril Dargazanli, Federico Cagnazzo, Alain Bonafé, Grégory Gascou, Pierre-Henri Lefèvre, Imad Derraz, Carlos Riquelme, Caroline Arquizan, Nicolas Gaillard, Isabelle Mourand, Lucas Corti.
TITAN investigators
Mohammad Anadani, Alejandro M. Spiotta, Ali Alawieh, Francis Turjman, Michel Piotin, Diogo C. Haussen, Raul G. Nogueira, Panagiotis Papanagiotou, Adnan H. Siddiqui, Bertrand Lapergue, Franziska Dorn, Christophe Cognard, Marc Ribo, Marios N. Psychogios, Marc Antoine Labeyrie, Mikael Mazighi, Alessandra Biondi, René Anxionnat, Serge Bracard, Sébastien Richard, Benjamin Gory, Jonathan Andrew Grossberg, Adrien Guenego, Julien Darcourt, Isabelle Vukasinovic, Elisa Pomero, Jason Davies, Leonardo Renieri, Corentin Hecker, Maria Muchada Muchada, Arturo Consoli, Georges Rodesch, Emmanuel Houdart, Raymond Turner, Aquilla Turk, Imran Chaudry, Johanna Lockau, Andreas Kastrup, Raphaël Blanc, Hocine Redjem, Daniel Behme, Hussain Shallwani, Maurer Christopher, Gioia Mione, Lisa Humbertjean, Jean-Christophe Lacour, François Zhu, AnneLaure Derelle, Romain Tonnelet, Liang Liao.
ACKNOWLEDGMENTS
References
Figure 2.
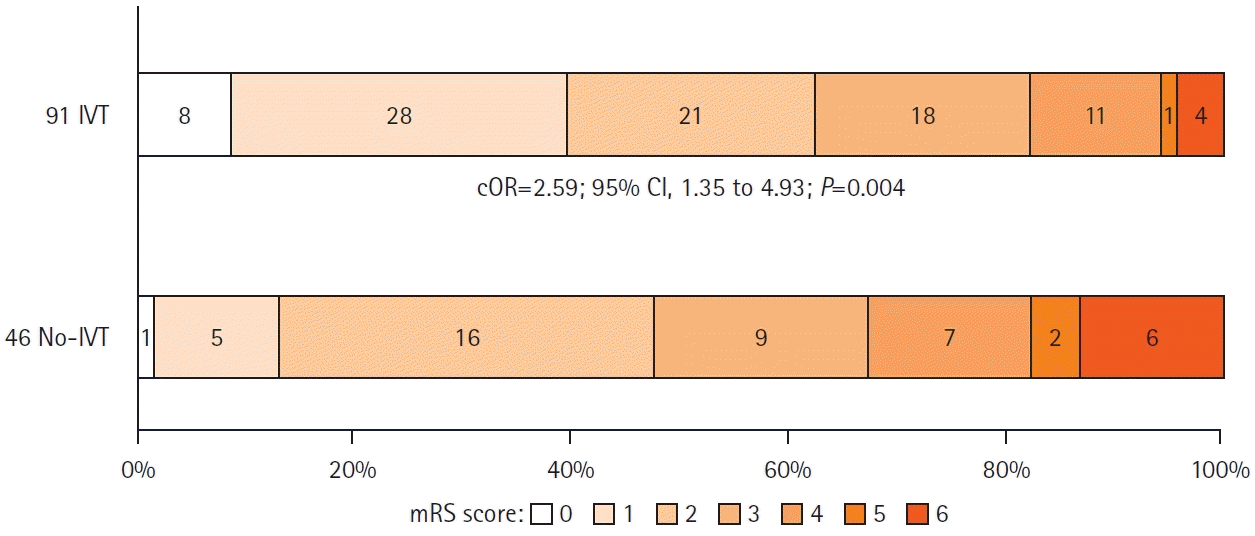
Table 1.
| Characteristic |
Intravenous thrombolysis |
P | ASD (%) | |
|---|---|---|---|---|
| No (n=50) | Yes (n=94) | |||
| Age (yr) | 52.0±12.9 | 51.9±11.0 | 0.96 | 1.0 |
| Men | 33/50 (66.0) | 62/93 (66.7) | 0.94 | 1.4 |
| Medical history | ||||
| Hypertension | 17/45 (37.8) | 28/91 (30.8) | 0.41 | 14.8 |
| Diabetes mellitus | 2/46 (4.3) | 5/91 (5.5) | 1.00 | 5.3 |
| Hypercholesterolemia | 3/45 (6.7) | 18/91 (19.8) | 0.046 | 39.5 |
| Current smoking | 17/44 (38.6) | 22/89 (24.7) | 0.097 | 30.3 |
| Current stroke event | ||||
| NIHSS score* | 17 (13–19) | 15 (10–20) | 0.59 | 9.5 |
| ASPECTS† | 7 (5–8) | 7 (6–8) | 0.42 | 14.6 |
| 0 | 0/45 (0.0) | 1/86 (1.2) | ||
| 2 | 1/45 (2.2) | 1/86 (1.2) | ||
| 3 | 1/45 (2.2) | 2/86 (2.3) | ||
| 4 | 6/45 (13.3) | 2/86 (2.3) | ||
| 5 | 5/45 (11.1) | 15/86 (17.4) | ||
| 6 | 7/45 (15.6) | 10/86 (11.6) | ||
| 7 | 9/45 (20.0) | 20/86 (23.3) | ||
| 8 | 7/45 (15.6) | 16/86 (18.6) | ||
| 9 | 3/45 (6.7) | 10/86 (11.6) | ||
| 10 | 6/45 (13.3) | 9/86 (10.5) | ||
| Initial cerebral imaging | 0.67 | 7.6 | ||
| MRI | 27/48 (56.3) | 51/85 (60.0) | ||
| CT scan | 21/48 (43.8) | 34/85 (40.0) | ||
| Onset to imaging (min)‡ | 127 (81–217) | 105 (78–169) | 0.19 | 25.8 |
| Endovascular treatment | ||||
| Site of intracranial occlusion | 0.068 | 45.7 | ||
| M1-MCA | 28/50 (56.0) | 61/94 (64.9) | ||
| M2-MCA | 11/50 (22.0) | 7/94 (7.4) | ||
| Intracranial ICA | 11/50 (22.0) | 25/94 (26.6) | ||
| Recanalized occlusion | 0/50 (0.0) | 1/94 (1.1) | ||
| Right-sided infarct | 21/46 (45.7) | 40/87 (46.0) | 0.97 | 0.7 |
| Onset to groin puncture (min)§ | 211 (165–312) | 226 (179–290) | 0.70 | 9.5 |
| General anesthesia | 18/48 (37.5) | 36/93 (38.7) | 0.89 | 2.5 |
| First line intracranial thrombectomy | 0.81 | 15.2 | ||
| SR | 15/48 (31.3) | 29/86 (32.6) | ||
| CA | 23/48 (47.9) | 35/86 (40.7) | ||
| SR+CA | 10/48 (20.8) | 22/86 (25.6) | ||
| Others | 0/48 (0.0) | 1/86 (1.2) | ||
| Cervical ICA stenting | 24/50 (48.0) | 45/94 (47.9) | 0.99 | 0.3 |
| Antiplatelet medication during procedure | 18/50 (36.0) | 37/94 (39.4) | 0.69 | 6.9 |
Values are presented as mean±standard deviation, number/total number (%), or median (interquartile range).
ASD, absolute standardized difference; NIHSS, National Institutes of Health Stroke Scale; ASPECTS, Alberta Stroke Program Early CT Score; MRI, magnetic resonance imaging; CT, computed tomography; MCA, middle cerebral artery; ICA, intracranial carotid artery; SR, stent retriever; CA, contact aspiration.
Table 2.
| Outcome |
Intravenous thrombolysis |
Unadjusted |
Adjusted†† |
||||
|---|---|---|---|---|---|---|---|
| No (n=50) | Yes (n=94) | Effect size (95% CI) | P | Effect size (95% CI) | P | ||
| Angiographic outcomes | |||||||
| Successful reperfusion | 32/50 (64.0) | 78/94 (83.0) | 2.74 (1.24 to 6.04)* | 0.012 | 2.63 (1.12 to 6.15)* | 0.025 | |
| First pass effect | 14/50 (28.0) | 33/94 (35.1) | 1.39 (0.65 to 2.95)* | 0.39 | 1.31 (0.87 to 1.42)* | 0.33 | |
| Groin puncture to reperfusion (min)† | 70 (40 to 87)‡ | 72 (50 to 89)‡ | 0.07 (–0.18 to 0.32)§ | 0.56 | 0.11 (–0.14 to 0.35)§ | 0.38 | |
| Number of passes | 2 (1 to 3)‡ | 2 (1 to 3)‡ | 1.10 (0.57 to 2.10)∥ | 0.78 | 1.38 (0.70 to 2.68)∥ | 0.35 | |
| Procedural complications‡‡ | 9/50 (18.0)¶ | 9/94 (9.6)¶ | 0.48 (0.17 to 1.31)* | 0.15 | 0.46 (0.15 to 1.33)* | 0.15 | |
| Clinical outcomes | |||||||
| 90-day favorable outcome | 22/46 (47.8) | 57/91 (62.6) | 1.83 (0.89 to 3.75)* | 0.099 | 1.69 (0.80 to 3.54)* | 0.16 | |
| 90-day excellent outcome | 6/46 (13.0) | 36/91 (39.6) | 4.36 (1.67 to 11.34)* | 0.003 | 4.23 (1.60 to 11.18)* | 0.004 | |
| 90-day mortality | 6/46 (13.0) | 4/91 (4.4) | 0.32 (0.08 to 1.12)* | 0.079 | 0.43 (0.11 to 1.63)* | 0.21 | |
| 24-hour change in NIHSS | 0.2 (–2.2 to 2.7)** | 3.2 (1.4 to 5.0)** | 2.9 (–0.1 to–5.9)†† | 0.056 | 3.6 (0.6 to 4.6)†† | 0.018 | |
| 24-hour change in ASPECTS | 1.8 (1.2 to 2.4)∥ | 1.1 (0.6 to 1.5)∥ | –0.8 (–1.5 to –0.1)** | 0.028 | –0.8 (–1.6 to –0.03)** | 0.040 | |
| Day-1 patency of extracranial ICA | 14/37 (37.8) | 23/58 (39.7) | 1.08 (0.46 to 2.52)* | 0.86 | 0.69 (0.26 to 1.79)* | 0.44 | |
| Any ICH | 24/39 (61.5) | 29/70 (41.4) | 0.44 (0.19 to 0.99)* | 0.046 | 0.41 (0.17 to 0.96)* | 0.042 | |
| Parenchyma hematoma | 7/39 (18.0) | 6/70 (8.6) | 0.43 (0.13 to 1.38)* | 0.16 | 0.38 (0.11 to 1.29)* | 0.14 | |
| sICH | 7/47 (14.8) | 4/94 (4.3) | 0.25 (0.07 to 0.92)* | 0.036 | 0.21 (0.05 to 0.80)* | 0.021 | |
Successful reperfusion was defined as modified Thrombolysis In Cerebral Infarction (mTICI) 2b/2c/3 at the end of endovascular procedure; first pass effect as mTICI 2b/2c/3 after first pass, favorable outcome as 90-day modified Rankin Scale (mRS) 0–2 and excellent outcome as 90-day mRS 0–1.
CI, confidence interval; NIHSS, National Institutes of Health Stroke Scale; ASPECTS, Alberta Stroke Program Early CT Score; ICA, intracranial carotid artery; ICH, intracranial hemorrhage; sICH, symptomatic intracranial hemorrhage.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download