Introduction
Methods
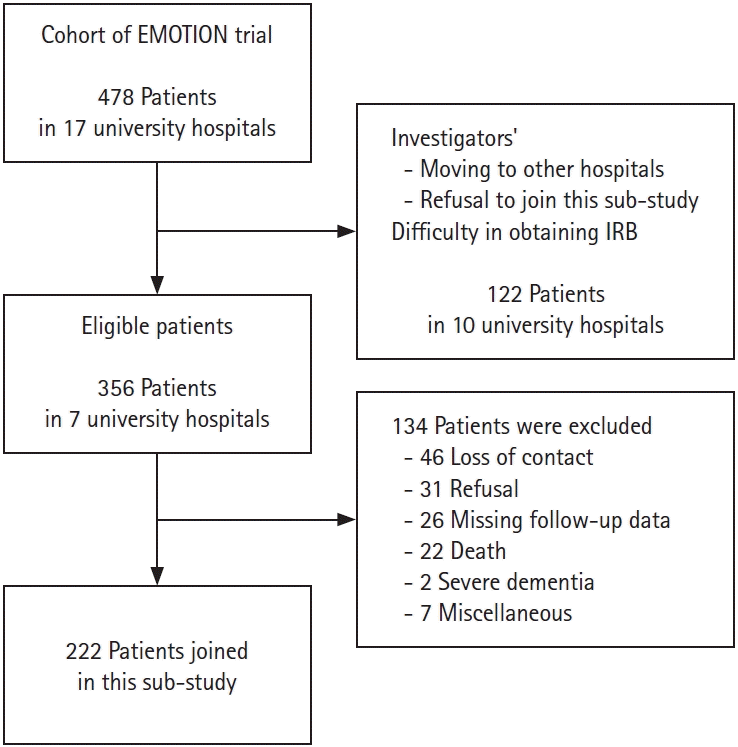
Study design and participants
Assessments
Data obtained from EMOTION trial
Long-term follow-up assessment
Statistical analysis
Results
Table 1.
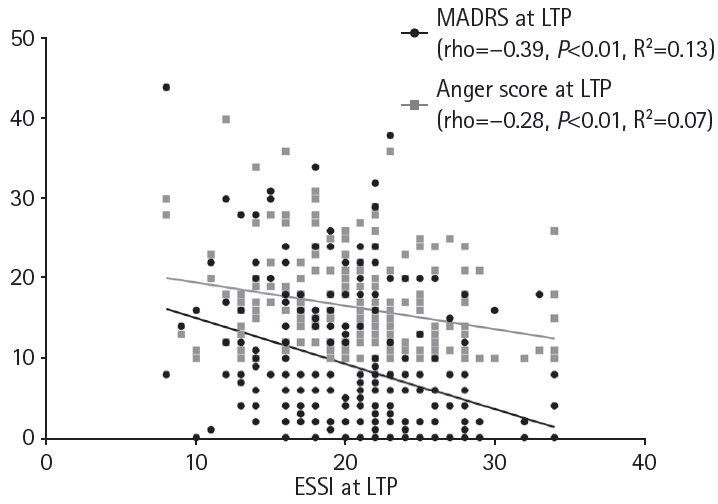
Figure 2.
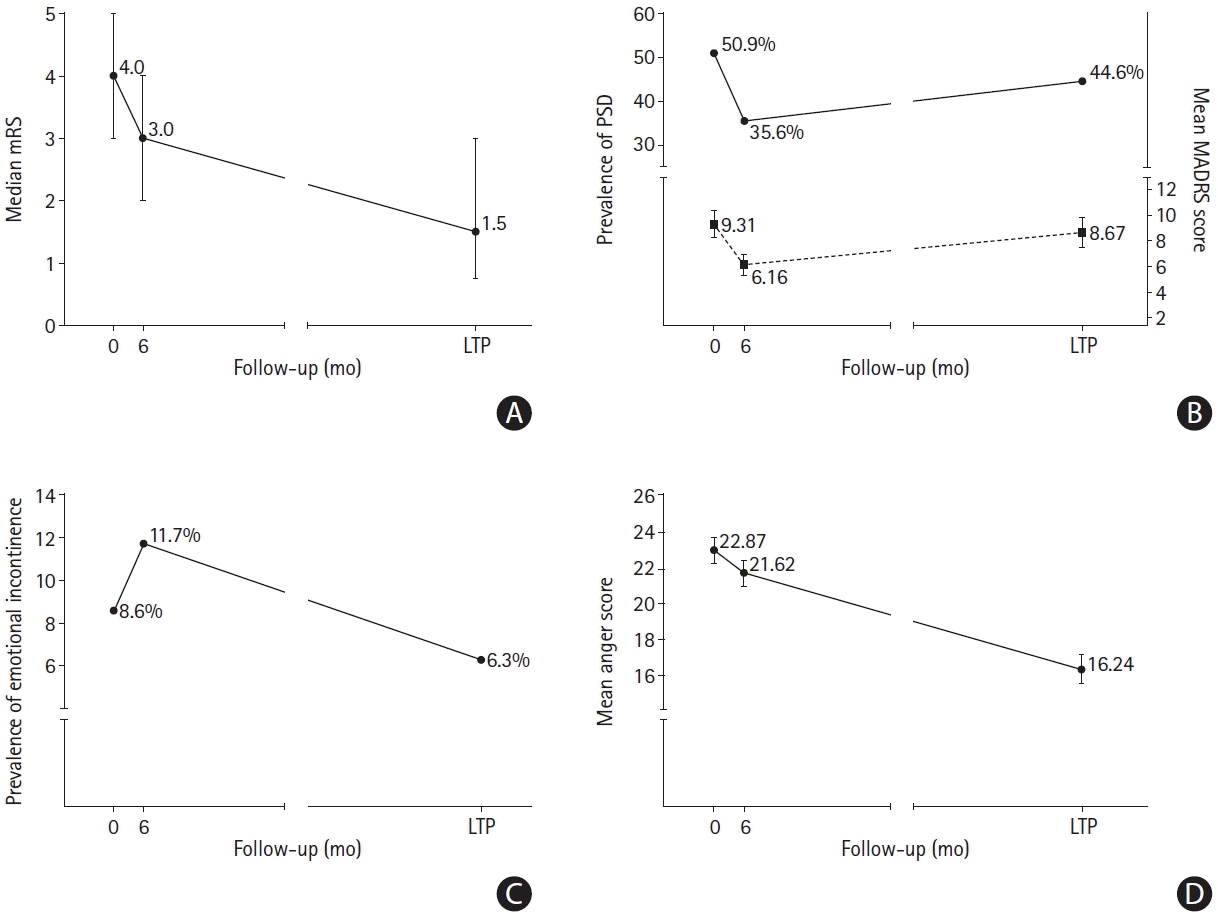
Table 2.
Logistic regression test for binary variables (PSD, PSEI) and linear regression test for continuous variable (anger score). Beta refers to the standardized beta coefficient of the linear regression test. The PSD, PSEI, and anger score at 6 months post-stroke were used to identify significant factors.
PSD, post-stroke depression; PSEI, post-stroke emotional incontinence; OR, odds ratio; CI, confidence interval; LTP, long-term period; MADRS, MontgomeryÅsberg depression rating scale; NIHSS, National Institutes of Health Stroke Scale.
Table 3.
| Variable |
6 Months post-stroke |
LTP |
||
|---|---|---|---|---|
| OR or beta (95% CI) | P | OR or beta (95% CI) | P | |
| PSD* | ||||
| Age (yr) | 0.97 (0.94 to 1.00) | 0.07 | 1.02 (0.99 to 1.04) | 0.20 |
| Female sex | 2.01 (1.01 to 3.98) | 0.05 | 1.23 (0.66 to 2.27) | 0.52 |
| Diabetes | 1.45 (0.75 to 2.82) | 0.27 | 1.07 (0.59 to 1.94) | 0.82 |
| Hyperlipidemia | 1.07 (0.55 to 2.07) | 0.84 | 2.22 (1.23 to 3.98) | 0.01 |
| Baseline PSD | 8.66 (4.23 to 17.73) | <0.01 | 2.81 (1.57 to 5.04) | <0.01 |
| Baseline NIHSS | 1.17 (1.04 to 1.32) | 0.01 | 1.10 (0.99 to 1.23) | 0.07 |
| PSEI* | ||||
| Lesion side, left | 1.16 (0.48 to 2.79) | 0.74 | 0.22 (0.06 to 0.85) | 0.03 |
| Baseline PSEI | 4.93 (1.68 to 14.51) | <0.01 | 4.84 (1.25 to 18.78) | 0.02 |
| Baseline NIHSS | 1.24 (1.07 to 1.43) | <0.01 | 1.19 (0.99 to 1.42) | 0.06 |
| Anger score† | ||||
| Age (yr) | –0.14 (–0.25 to –0.02) | 0.02 | –0.08 (–0.22 to 0.05) | 0.24 |
| Female sex | 0.05 (–0.11 to 0.21) | 0.54 | –0.09 (–0.27 to 0.10) | 0.38 |
| Hypertension | 0.00 (–0.11 to 0.11) | 0.97 | 0.10 (–0.04 to 0.23) | 0.16 |
| Hyperlipidemia | –0.01 (–0.12 to 0.10) | 0.85 | 0.11 (–0.02 to 0.24) | 0.11 |
| Smoking | –0.01 (–0.16 to 0.15) | 0.93 | 0.02 (–0.16 to 0.21) | 0.80 |
| Lesion side, left | 0.01 (–0.10 to 0.12) | 0.83 | –0.09 (–0.22 to 0.04) | 0.17 |
| Baseline anger score | 0.61 (0.49 to 0.72) | <0.01 | 0.20 (0.07 to 0.34) | <0.01 |
| Baseline NIHSS | 0.02 (–0.09 to 0.13) | 0.76 | 0.14 (0.01 to 0.27) | 0.04 |
Logistic regression test for binary variables (PSD, PSEI) and linear regression test for continuous variable (anger score). Beta refers to the standardized beta coefficient of the linear regression test. The PSD, PSEI, and anger score at 6 months post-stroke were used to identify significant factors.
PSD, post-stroke depression; PSEI, post-stroke emotional incontinence; LTP, long-term period; OR, odds ratio; CI, confidence interval; NIHSS, National Institutes of Health Stroke Scale.
Table 4.
| Variable |
Simple test |
Adjusted test |
||
|---|---|---|---|---|
| OR or beta (95% CI) | P | OR or beta (95% CI) | P | |
| PSD at LTP* | ||||
| Age (yr) | 0.98 (0.95 to 1.01) | 0.23 | ||
| Female sex | 1.53 (0.74 to 3.18) | 0.25 | ||
| Baseline PSD | 3.12 (1.56 to 6.24) | <0.01 | ||
| Baseline NIHSS | 0.90 (0.79 to 1.03) | 0.13 | ||
| Regular clinic visit | 3.50 (1.11 to 11.03) | 0.03 | ||
| Antidepressant use | 3.38 (1.02 to 11.18) | 0.05 | ||
| Recurrent stroke | 0.46 (0.09 to 2.42) | 0.36 | ||
| mRS | 2.12 (1.67 to 2.70) | <0.01 | 2.16 (1.54 to 3.02) | <0.01 |
| Low social support | 3.46 (1.94 to 6.16) | <0.01 | 4.12 (1.97 to 8.66) | <0.01 |
| PSEI at LTP* | ||||
| Age (yr) | 0.98 (0.92 to 1.04) | 0.47 | ||
| Female sex | 1.51 (0.43 to 5.25) | 0.52 | ||
| Baseline PSEI | 3.94 (0.92 to 16.85) | 0.06 | ||
| Baseline NIHSS | 1.12 (0.90 to 1.40) | 0.32 | ||
| MADRS at LTP | 1.07 (0.99 to 1.15) | 0.08 | ||
| Regular clinic visit | 0.31 (0.03 to 3.61) | 0.35 | ||
| Antidepressant use | 1.68 (0.34 to 8.22) | 0.52 | ||
| Recurrent stroke | 0.52 (0.04 to 7.74) | 0.64 | ||
| mRS | 1.39 (0.95 to 2.04) | 0.09 | 0.96 (0.50 to 1.84) | 0.90 |
| Low social support | 3.63 (1.17 to 11.23) | 0.03 | 2.01 (0.53 to 7.69) | 0.31 |
| Anger score at LTP† | ||||
| Age (yr) | –0.10 (–0.23 to 0.03) | 0.14 | ||
| Female sex | –0.06 (–0.18 to 0.07) | 0.38 | ||
| Baseline anger score | 0.22 (0.10 to 0.34) | <0.01 | ||
| Baseline NIHSS | 0.06 (–0.07 to 0.18) | 0.39 | ||
| MADRS | 0.45 (0.31 to 0.59) | <0.01 | ||
| Regular clinic visit | 0.11 (–0.01 to 0.23) | 0.07 | ||
| Antidepressant use | 0.04 (–0.08 to 0.17) | 0.52 | ||
| Recurrent stroke | 0.04 (–0.07 to 0.16) | 0.48 | ||
| mRS | 0.11 (–0.02 to 0.24) | 0.10 | –0.14 (–0.30 to 0.02) | 0.09 |
| Low social support | 0.25 (0.12 to 0.38) | <0.01 | 0.14 (0.02 to 0.26) | 0.03 |
Logistic regression test for binary variables (PSD, PSEI) and linear regression test for continuous variable (anger score). Beta refers to the standardized beta coefficient of the linear regression test.
mRS, modified Rankin Scale; LTP, long-term period; PSD, post-stroke depression; PSEI, post-stroke emotional incontinence; OR, odds ratio; CI, confidence interval; NIHSS, National Institutes of Health Stroke Scale; MADRS, Montgomery-Åsberg depression rating scale.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download