This article has been
cited by other articles in ScienceCentral.
Abstract
Wernicke’s encephalopathy is a reversible but potentially critical disease caused by thiamine deficiency. Most patients complain of symptoms such as ophthalmoplegia, ataxia and confusion. Heavy alcohol drinking is commonly associated with the disease, but other clinical conditions also can provoke it. In pregnant women, hyperemesis gravidarum can lead to the depletion of body thiamine due to poor oral intake and a high metabolic demand. We report a case of Wernicke’s encephalopathy following hyperemesis gravidarum in a 36-year-old female at 20 weeks of pregnancy, who visited our hospital because of shock with vaginal bleeding. This case suggests that although the initial presentation may include atypical symptoms (e.g., shock or bleeding), Wernicke’s encephalopathy should be considered, and thiamine replacement should be performed in pregnant women with neurologic symptoms and poor oral intake.
Go to :

Keywords: acute kidney injury, hyperemesis gravidarum, Wernicke encephalopathy
Nausea and vomiting are common symptoms in early pregnancy during the first trimester.[
1] Hyperemesis gravidarum is a severe complication of pregnancy characterized by intractable nausea, vomiting, dehydration, and weight loss, that affects 0.3–2.0% of pregnant women.[
2] If hyperemesis gravidarum is not well controlled, it can lead to Wernicke’s encephalopathy. Wernicke’s encephalopathy is an acute neuropsychiatric thiamine deficiency disorder associated with alcoholism and malnutrition. The syndrome was first identified by Carl Wernicke in 1881 who described a disorder characterized by a triad of signs that included a change in mental status, ocular abnormalities, and motor problems. He termed the disorder
polioencephalitis hemorrhagica superioris.[
3] We report here a life-threatening case of Wernicke’s encephalopathy caused by hyperemesis gravidarum in a 36-year-old woman.
Case Report
A 36-year-old woman in the second trimester of pregnancy (20 weeks gestation) was admitted to the emergency department of our hospital because of shock and vaginal bleeding. Her diet was so inadequate and she had lost 20 kg over a two-month period because of severe hyperemesis and vomiting. She had a history of full-term delivery 4 years prior and had suffered from hyperemesis at that time. On admission, her initial vital signs were as follows: blood pressure 45/25 mmHg, pulse rate 140 beats/min, respiration rate 18 breaths/min, and body temperature 37.4°C.
On physical examination, she looked chronically ill. She had clear breath sounds on chest auscultation and mild suprapubic tenderness on abdominal examination. Her neurologic examination showed confusion (Glasgow Coma Scale: eye open 4, best verbal response 1, best motor response 5), hypophonia, gaze-evoked nystagmus (right side), diffuse muscle atrophy, and generalized weakness (Medical Research Council grade II).
The results of a complete blood cell count showed a white blood cell count of 9,400/μL, hemoglobin level of 9.5 g/dL, and platelet count of 304,000/μL. She had coagulopathy with a prothrombin time of 48.5%. Kidney function and electrolyte results suggested renal failure based on the following values: blood urea nitrogen, 247 mg/dL; creatinine, 5.70 mg/dL; phosphorus, 8.8 mg/dL; and calcium, 8.1 mg/dL. Liver function tests showed isolated hyperbilirubinemia with a total bilirubin value of 7.2 mg/dL and a direct bilirubin value of 6.6 mg/dL.
Intravenous saline loading and a transfusion were administered, but vaginal bleeding continued. Gynecological ultrasound findings showed that the fetus was small for the gestational age and had severe bradycardia (the beats per minute were not noted). The patient was admitted to the medical intensive care unit, and then she received an induced artificial abortion. The vaginal bleeding reduced, and her blood pressure increased to 106/59 mmHg after the abortion. However, although she received 4 L of a saline infusion and five bags of red blood cells within the initial 12 h of admission, her urine output was <10 mL/h; hence, we initiated continuous renal replacement therapy (CRRT).
After the vital signs were stabilized, she received intravenous glucose with electrolytes 18 h after admission. Her abnormal laboratory values and urine output gradually improved, and CRRT was stopped on hospital day 3. We initially thought her neurologic symptoms and signs were due to uremic encephalopathy. However, the confusion, nystagmus, hypophonia, and polyneuropathy did not improve despite these treatments. We consulted her to the neurology department for a magnetic resonance imaging (MRI) study of the brain because of suspicion of Wernicke’s encephalopathy. The MRI showed bilateral symmetric edematous high signal changes in the medio-posterior thalami, fornices, and mammillary bodies (
Fig. 1). Nerve conduction studies were also performed because of the polyneuropathy, and the findings were compatible with combined critical illness polyneuropathy (CIP) and compressive peroneal neuropathy.
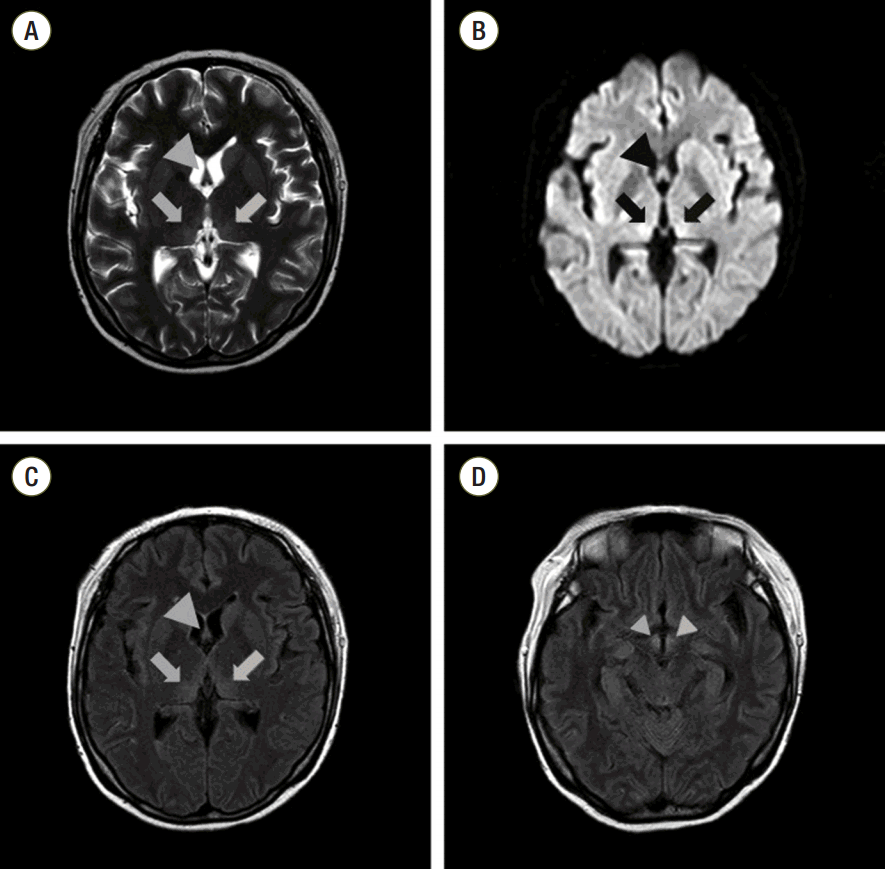 | Fig. 1.Initial brain magnetic resonance imaging findings: T2-weighted imaging and diffusion-weighted imaging showed bilateral symmetric edematous high-signal changes in medio-posterior thalami (arrow) and fornices (large arrowhead) (A, B). Fluid-attenuated inversion recovery imaging showed high-signal-intensity lesions in medio-posterior thalami (arrow), fornices (large arrowhead) and mammillary bodies (small arrowhead) (C, D). (A) T2-weighted imaging of both medio-posterior thalami and fornices. (B) Diffusion-weighted imaging of both medio-posterior thalami and fornices. (C) Fluid-attenuated inversion recovery imaging of both medio-posterior thalami and fornices. (D) Fluid-attenuated inversion recovery imaging of both mammillary bodies. 
|
On the basis of her clinical signs, radiological findings, and nerve conduction studies, we diagnosed CIP and compressive peroneal neuropathy in addition to Wernicke’s encephalopathy. We treated her with 500 mg of thiamine intravenously three times daily for 3 days.[
4] Her confusion and neurologic symptoms markedly improved after treatment. We continued 50 mg of thiamine three times daily until the confusion and neurologic symptoms had sufficiently stabilized. Although the other symptoms had resolved, she still complained of lower limb weakness because the combined CIP and compressive peroneal neuropathy had not completely resolved. She was therefore transferred to the department of rehabilitation medicine for ambulatory remedial exercises. In the following 4 weeks, her symptoms improved and she was able to walk using a walker upon discharge.
Go to :

Discussion
Wernicke’s encephalopathy is caused by thiamine deficiency. It is most common in alcoholics, but it also occurs in patients with malnutrition due to hyperemesis, starvation, renal replacement therapy, malignancy, and gastric surgery.[
5] Severe thiamine deficiency can occur in pregnant women with hyperemesis gravidarum resulting from a combination of poor dietary intake, frequent vomiting, and increased metabolic demands. However, as the economy has developed and people are now well-educated in Korea, Wernicke’s encephalopathy rarely occurs in pregnant women nowadays.[
6]
The diagnosis of Wernicke’s encephalopathy may be difficult because of the high rate at which patients present with nonspecific symptoms and neurologic signs.[
4] There is no specific laboratory test to diagnose Wernicke’s encephalopathy. Serum thiamine levels do not precisely reflect the total body thiamine status and they can be within normal range in Wernicke’s encephalopathy.[
7] In our patient, we checked the thiamine level (84.6 nmol/L) using high performance liquid chromatographic analysis, and it was within normal range (59–213 nmol/L).
Brain MRI is extensively used for the initial diagnosis of Wernicke’s encephalopathy and to rule out other differential diagnoses. Typical MRI findings in Wernicke’s encephalopathy include symmetrically increased signal changes in the medial aspect of thalami; mammillary bodies; periaqueductal lesions and mesencephalic tegmentum on T2-weighted images; and fluid attenuated inversion recovery images.[
7,
8]
A diagnosis of Wernicke’s encephalopathy was delayed in our case. The patient had a history of mental changes with acute kidney injury. We initially considered her mentality was related to a metabolic encephalopathy such as uremic encephalopathy, because she presented with shock, electrolyte abnormalities, and high blood urea nitrogen. However, her condition did not improve despite CRRT and effective medical management for shock. When we administered intravenous dextrose without thiamine, her confusion was aggravated. After considering her clinical manifestations, radiologic findings, and response to thiamine therapy, we were able to diagnose her condition as Wernicke’s encephalopathy.[
9]
Patients with Wernicke’s encephalopathy should be treated immediately with a minimum of 500 mg of thiamine dissolved in 100 mL of saline administered intravenously three times per day for 2–3 days. After assessing the response, 250 mg of thiamine per day should be given intravenously for 3–5 days or until the clinical signs resolve.[
4] Glucose supplementation without thiamine can increase the thiamine requirement so care is needed.[
7] If patients with Wernicke’s encephalopathy receive inappropriate or delayed treatment, it can be potentially fatal and provoke several problems such as neurologic sequelae in the mother[
10] and miscarriage, preterm birth, and intrauterine growth retardation in the fetus.[
11]
In conclusion, Wernicke’s encephalopathy can present with nonspecific symptoms and signs or in combination with other medical conditions. Therefore, although the initial presentation is shock or bleeding, Wernicke’s encephalopathy should be considered when pregnant women with severe hyperemesis, vomiting, and weight loss complain of neurologic symptoms. Treatment for shock and other symptoms as well as empirical thiamine replacement must be included in the initial management of these patients.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download