This article has been
cited by other articles in ScienceCentral.
Abstract
A 71-year-old male initially presented with vocal cord palsy and underwent tracheostomy. After thorough examination, urogenital dysfunction, orthostatic hypotension, and Parkinsonism were found, which led to the diagnosis of multiple system atrophy (MSA). After the tracheostomy, bi-level positive airway pressure ventilation was required during the night due to nocturnal hypoxemia. Nighttime hypoxemia is related to central sleep apnea, which is one of the manifestations of MSA. This is the first case of MSA manifested by bilateral vocal cord palsy as an initial sign in Korea. This case supports the notion that MSA should be taken into consideration when vocal cord paralysis is observed.
Go to :

Keywords: airway obstruction, multiple system atrophy, parkinsonism, vocal cord paralysis
Multiple system atrophy (MSA) is a progressive neurodegenerative disorder presenting with a variety of symptoms, for example parkinsonism, cerebellar dysfunction, corticospinal dysfunction and autonomic nervous system abnormality. Vocal cord abductor paralysis is a life-threatening complication that can lead to sudden death at night and is considered a poor prognostic factor in MSA.[
1]
We describe the case of a patient who presented with dyspnea caused by vocal cord paralysis as part of the initial symptoms of MSA, which was later diagnosed. This is the first case report of vocal cord palsy caused by MSA in Korea.
Case Report
On August 6th, 2013, a 71-year-old man was admitted to the emergency room (ER) presenting with symptoms of fever and dyspnea for three days. He had a medical history of benign prostatic hyperplasia, had undergone transurethral prostatectomy two years ago (January 11, 2012), and reported hoarseness and snoring. The hoarseness, which brought him to a ENT clinic, had developed 2 years ago (January 19, 2012). Although a diagnosis of bilateral vocal cord paresis with unknown cause was made at that time, no intervention was made and the next visit was arranged for 3 months, since the width between his two vocal cords was sufficiently wide to permit him to breathe (4 mm). However the patient had not visited the clinic as scheduled.
In the ER, his vital signs were 135/73 mmHg of blood pressure, 98 beats/min of pulse rate, 26 beats/min of respiratory rate, and 38.7℃ of body temperature. Total leucocytes were elevated to 10,100 /μL. Arterial blood gas analysis on 3 LPM of oxygen by nasal prongs was pH 7.26, PaCO
2 84 mmHg, PaO
2 65 mmHg, and HCO
3 - 38 mmol/L. The width between his vocal cords was reduced by up to 1 mm compared with that of 2 years ago. Intubation followed, and a tracheostomy was performed. A bronchoscopy examination after the tracheostomy revealed vocal cord paralysis (
Fig. 1), and sepsis was associated with a urinary tract infection. Urinary retention with straining to void had persisted even after transurethral prostatectomy for benign prostatic hyperplasia two years earlier to visit ER. No decrease in muscle strength or prominent symptoms of Parkinson’s disease was seen. His general condition improved, with tolerable oxygen saturation in room air, and he was discharged. The tracheotomy was maintained.
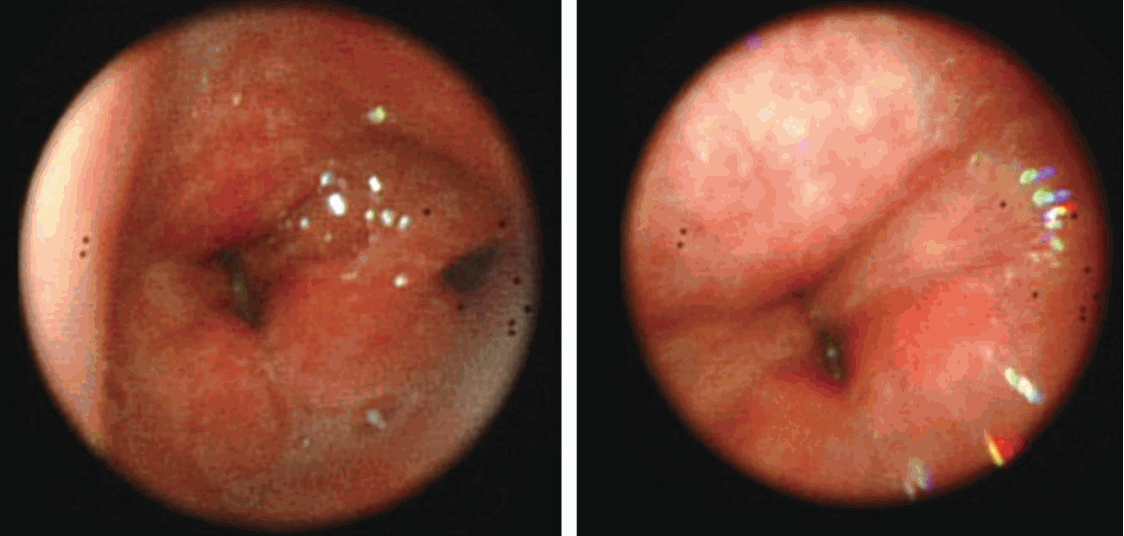 | Fig. 1.Absence of movement of the vocal cords during bronchoscopy performed after tracheal intubation. 
|
Four months later (December 2nd, 2013), the patient was revisited with an altered mental state that had developed the previous day. His oxygen saturation was 20% at arrival to the hospital. His mental status rapidly improved after mechanical ventilatory support. After admission to hospital with pneumonia and recurrent hypercapneic oxygenation failure, BiPAP was applied. Bilateral diaphragmatic movement was 8-10 mm during normal breathing and 39-35 mm on deep inhalation, and no diaphragmatic dysfunction was evident. Postural tremor, bradykinesia and rigidity of both fingers and hands were revealed by neurologic examination. The patient also had a history of short step gait and bent posture which had progressed during the previous two years.
After a thorough review of the patient’s medical history it was discovered that he had been diagnosed with orthostatic hypotension and there had been a strong suspicion of sleep apnea. Transcranial doppler sonography supported a diagnosis of orthostatic hypotension causing systolic blood pressure to fall from 167 mmHg to 96 mmHg within nine minutes of standing. There was no significant change in mean velocity. A diagnosis of multiple system atrophy with prominent parkinsonism (MSA-parkinsonian type, MSA-P) was made after obtaining evidence of orthostatic hypotension, possible sleep apnea, urinary tract disorder, neurological abnormality with postural tremor, and history of short gait step and bent posture. BiPAP was applied at night because of fear of central sleep apnea due to the degeneration of the respiratory centers in the brain stem in MSA-P. This had more of an effect on the involuntary breathing during the night than the voluntary breathing.
Image findings supported the diagnosis of MSA-P. Dopamine transporter (DAT) positron emission tomography (
18F-fluorinated-N-3-fluoropropyl-2-b-carboxymethoxy-3-b-[4-iodophenyl]nortropane Brain PETCT) revealed a decreased binding of dopamine transporters to the bilateral putamen, relative preservation of DAT biding in caudate nucleus. Previously taken cross-sectional imaging of brain magnetic resonance imaging (MRI) T2 showed the hypointensity and fuzzy dorsolateral boundaries of the posterior putamen, and subtle increase in intensity in the lateral putaminal rim, which could be an early finding of MSA in certain clinical circumstances. There was no clinically significant focal lesion in brain stem or cerebellum (
Fig. 2). EMG and NCV were performed to rule out other neuromuscular disorders and there were no specific findings except for left sacral radiculopathy. After the diagnosis of MSA-P, the patient was discharged with levodopa/carbidopa 100/25 mg 3 times a day. There seemed no significant response to levodopa, considering a report of his persistent bed-ridden status from a visiting nurse.
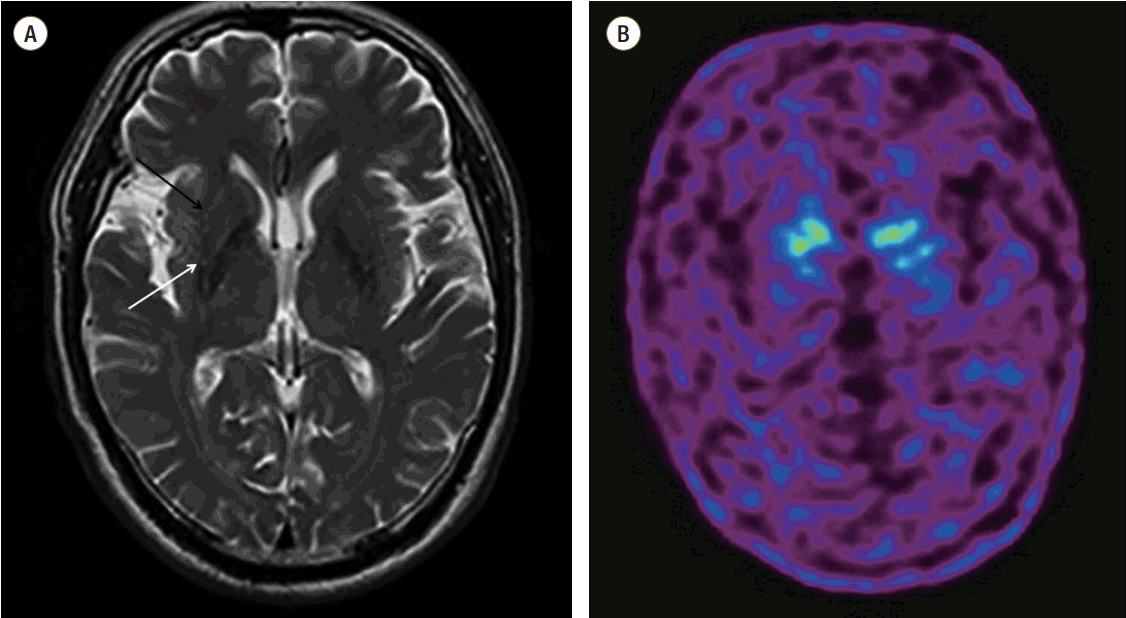 | Fig. 2.(A) T2-weighted axial images of brain MRI shows the hypointensity and fuzzy boundaries of the dorsolateral part of the posterior putamen (white arrow). The signal intensity appears to increase around the outward edge of the putamen (black arrow). (B) Dopamine transporter positron emission tomography image (F-18 FP-CIT Brain PETCT) shows reduced numbers of dopamine transporters in the bilateral putamen. 
|
Go to :

Discussion
According to revised diagnostic criteria set forth in the 2008 MSA consensus statement, a diagnosis of definite MSA is based upon postmortem pathology showing alphasynuclein- positive glial cytoplasmic inclusions with neurodegenerative changes in striatonigral or olivopontocerebellar structures.[
2] This case was presented by sporadic, progressive and over 30 years of age onset disease with autonomic failure involving urinary incontinence and orthostatic decreased of blood pressure and poorly levodopa-responsive parkinsonism such as bradykinesia with rigidity and tremor. These findings met the criteria of probable MSA. In addition, this patient showed atrophy of putamen on MRI and presynaptic nigrostriatal dopaminergic denervation on PET, which supported the diagnosis of MSA.
MSA is a sporadic and rapidly progressive neurodegenerative disorder that presents with autonomic failure in combination with parkinsonism or cerebellar ataxia.[
3] MSA is characterised by a variable combination of autonomic dysfunction, parkinsonism that is poorly responsive to le-vodopa, cerebellar ataxia, and pyramidal symptoms. The pathological features comprise neuronal loss in the basal ganglia, cerebellum, pons, inferior olivary nuclei, and spinal cord accompanied by gliosis.[
3]
Laryngeal stridor also occurs because of local inflammation or tumor, or from distant causes that affect the vagal nerves, such as upper thoracic or nasopharyngeal carcinoma. If such conditions have been excluded, central neurological causes should be considered.[
4] If vocal cord paralysis was manifested as an initial sign of the MSA, accurate early diagnosis is often difficult requiring long-term follow-up to make the diagnosis.[
5] This case also, at the time the tracheostomy, was not revealed the reason for the vocal cord paralysis. Four months later when the patient was re-admitted unconsciously, postural tremor, bradykinesia in both hands, and rigidity were detected on neurologic examination, and provided the clue for MSA. As such, the diagnosis of MSA should be considered before a diagnosis of idiopathic vocal cord paralysis is made.[
6]
Bilateral vocal fold paralysis generally presents at an advanced stage of the disease. However it can appear at an early stage, and may even be the only symptom. Stridor as an early manifestation has been demonstrated only in 4% of patients with probable MSA.[
4,
5,
7,
8] The pathogenesis of stridor remains still controversial.[
9,
10] One study indicate that contraction of laryngeal adductors during inspiration narrows the larynx leading to development of inspiratory flow limitation accompanied by stridor in patients with MSA.[
11] MSA related vocal cord paralysis could result from the disease-related damage to the pontomedullary respiratory center.[
12] There are some histological studies showing that the atrophy of laryngeal muscle, such as the posterior cricoarytenoid muscle, is the reason of the vocal cord paralysis in MSA patients.[
13,
14]
Urinary dysfunction may present years in advance of the typical neurological symptoms.[
15] In the present case, urinary dysfunction was already present when sleep apnea occurred. When the patient visited the urology outpatient clinic 3 years previously, he had been diagnosed with benign prostate hyperplasia. This made it difficult to suspect autonomic nervous system-induced urinary dysfunction. However, the persistence of urinary retention after transurethral prostate resection was likely to be due to autonomic nervous system dysfunction. In particular, urine storage dysfunction and sleep apnea are often associated with sudden death caused by paralysis of the abductor muscle at night. [
5,
16]
In conclusion, this is the first case of MSA manifested by bilateral vocal cord palsy as an initial sign in South Korea. It could be helpful for clinicians to consider MSA in differential diagnosis of the vocal cord palsy.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download