Introduction
Methods
Study population and stroke care
Data collection
MRI protocols and assessments
Statistical analysis
Figure 1.
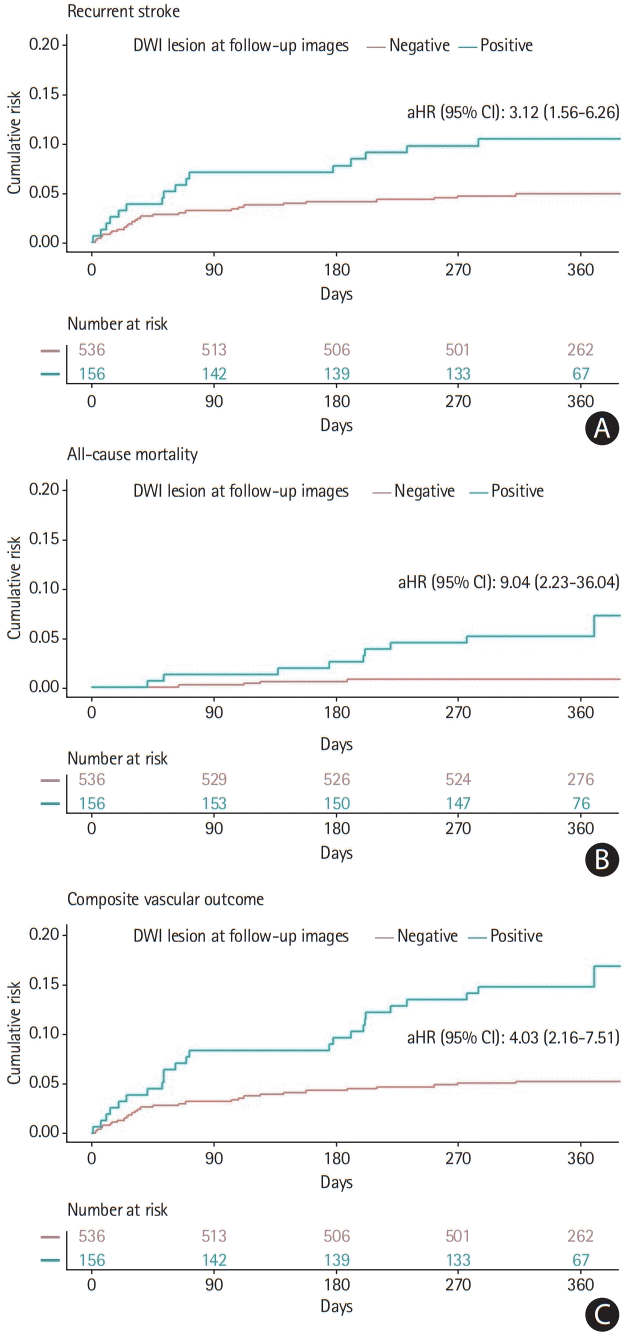
Table 1.
| Variable | Whole subjects (n=694) | Model development set (n=488) | External validation set (n=206) | P |
|---|---|---|---|---|
| Male sex | 404 (58.2) | 285 (58.4) | 119 (57.8) | 0.88 |
| Age at arrival (yr) | 62.9±13.7 | 62.7±13.4 | 63.4±14.4 | 0.52 |
| Height (cm) | 163.7±8.5 | 163.5±8.5 | 164.2±8.6 | 0.32 |
| Weight (kg) | 65.3±11.6 | 65.1±11.3 | 65.6±12.2 | 0.60 |
| BMI (kg/cm2) | 24.3±3.2 | 24.3±3.1 | 24.2±3.4 | 0.91 |
| Initial NIHSS* | 0.17 | |||
| 0 | 440 (63.4) | 318 (65.2) | 122 (59.2) | |
| 1 | 114 (16.4) | 75 (15.4) | 39 (18.9) | |
| ≥2 | 140 (20.2) | 95 (19.5) | 45 (21.8) | |
| Discharge NIHSS* | 0.69 | |||
| 0 | 517 (74.5) | 359 (73.6) | 158 (76.7) | |
| 1 | 99 (14.3) | 72 (14.8) | 27 (13.1) | |
| ≥2 | 78 (11.2) | 57 (11.7) | 21 (10.2) | |
| Pre-stroke mRS† | 0.89 | |||
| 0 | 649 (93.5) | 456 (93.4) | 193 (93.7) | |
| 1 | 23 (3.3) | 16 (3.3) | 7 (3.4) | |
| ≥2 | 22 (3.2) | 16 (3.3) | 6 (2.9) | |
| Post-stroke mRS† | 0.65 | |||
| 0 | 477 (68.7) | 332 (68.0) | 145 (70.4) | |
| 1 | 127 (18.3) | 89 (18.2) | 38 (18.4) | |
| ≥2 | 90 (13.0) | 67 (13.2) | 23 (11.2) | |
| Initial systolic BP | 154.3±27.1 | 154.7±27.2 | 153.4±26.8 | 0.57 |
| Initial diastolic BP | 85.5±16.2 | 85.8±16.5 | 84.9±15.5 | 0.49 |
| Clear onset | 563 (81.1) | 392 (80.3) | 171 (83.0) | 0.41 |
| Early neurologic deterioration | 23 (3.3) | 20 (4.1) | 3 (1.5) | 0.12 |
| Dysarthria | 308 (44.4) | 210 (43.0) | 98 (47.6) | 0.31 |
| Subjective hemiparesis | 368 (53.0) | 267 (54.7) | 101 (49.0) | 0.20 |
| Side of hemiparesis | 0.23 | |||
| None | 326 (47.0) | 221 (45.3) | 105 (51.0) | |
| Left | 191 (27.5) | 134 (27.5) | 57 (27.7) | |
| Right | 177 (25.5) | 133 (27.3) | 44 (21.4) | |
| Objective hemiparesis | 37 (5.3) | 24 (4.9) | 13 (6.3) | 0.57 |
| Objective arm weakness (MRC‡ grade) | ||||
| 5 | 640 (92.2) | 452 (92.6) | 188 (91.3) | 0.76 |
| <5 | 54 (7.8) | 36 (7.4) | 18 (8.7) | |
| Objective leg weakness (MRC‡ grade) | ||||
| 5 | 628 (90.5) | 439 (90.0) | 189 (91.7) | 0.37 |
| <5 | 66 (9.5) | 49 (10.0) | 17 (8.3) | |
| Dizziness | 164 (23.6) | 104 (21.3) | 60 (29.1) | <0.05 |
| Veering tendency | 65 (9.4) | 42 (8.6) | 23 (11.2) | 0.36 |
| Ataxia | 31 (4.5) | 23 (4.7) | 8 (3.9) | 0.78 |
| Hypesthesia | 138 (19.9) | 99 (20.3) | 39 (18.9) | 0.76 |
| Paresthesia | 82 (11.8) | 57 (11.7) | 25 (12.1) | 0.97 |
| Paresthesia & hypesthesia, simultaneously | 34 (4.9) | 22 (4.5) | 12 (5.8) | 0.59 |
| Aphasia | 42 (6.1) | 31 (6.4) | 11 (5.3) | 0.74 |
| Diplopia | 21 (3.0) | 17 (3.5) | 4 (1.9) | 0.40 |
| Duration | ||||
| >24 hr | 25 (3.6) | 22 (4.5) | 3 (1.4) | 0.09 |
| ≥1 and <24 hr | 329 (47.4) | 224 (45.9) | 105 (51.0) | |
| ≥10 min and <1 hr | 157 (22.6) | 116 (23.8) | 41 (19.9) | |
| <10 min | 105 (15.1) | 77 (15.8) | 28 (13.6) | |
| No data | 78 (11.2) | 49 (10.0) | 29 (14.1) | |
| Time from the first symptom onset to the initial MR image (hr) | 4.4 (2.0–10.5) | 4.5 (1.9–10.6) | 4.1 (2.2–10.2) | <0.05 |
| Time from the first symptom onset to the follow-up MR image (hr) | 61.0 (45.9–83.5) | 65.5 (49.3–89.1) | 50.7 (41.3–69.5) | <0.05 |
| Time from the initial MR image to the follow-up MR image (hr) | 51.5 (40.6–69.9) | 55.4 (43.0–75.1) | 44.8 (37.0–58.1) | <0.05 |
| Hypertension | 432 (62.2) | 311 (63.7) | 121 (58.7) | 0.25 |
| Diabetic mellitus | 164 (23.6) | 119 (24.4) | 45 (21.8) | 0.53 |
| Hyperlipidemia | 263 (37.9) | 178 (36.5) | 85 (41.3) | 0.27 |
| Atrial fibrillation | 61 (8.8) | 46 (9.4) | 15 (7.3) | 0.44 |
| Smoking | 0.83 | |||
| Current | 155 (22.3) | 112 (23.0) | 43 (20.9) | |
| Ex-smoker | 118 (17.0) | 83 (17.0) | 35 (17.0) | |
| None | 421 (60.7) | 293 (60.0) | 128 (62.1) | |
| Serum creatinine (mg/dL) | 0.88±0.50 | 0.88±0.53 | 0.89±0.44 | 0.78 |
| Hemoglobin (g/dL) | 13.9±1.7 | 13.9±1.7 | 13.9±1.6 | 0.79 |
| Total cholesterol level >240 mg/dL | 168.1±38.4 | 169.2±38.4 | 165.3±38.3 | 0.21 |
| Follow-up positive-conversion rate | 156 (22.5) | 111 (22.7) | 45 (21.8) | 0.87 |
Values are presented as mean±standard deviation, number (%), or median (interquartile range). Calculated using two-sample t-test for continuous variables or chi-square test for categorical variables.
BMI, body mass index; NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale; BP, blood pressure; MRC, Medical Research Council; MR, magnetic resonance.
Table 2.
| Outcome | Positive-conversion and covaries | Adjusted OR (95% CI) |
|---|---|---|
| Recurrent stroke | DWI-positive conversion | 3.12 (1.56–6.26) |
| Age | 1.03 (1.01–1.06) | |
| Male sex | 1.10 (0.57–2.11) | |
| Atrial fibrillation | 0.77 (0.30–1.99) | |
| Smoking | 3.13 (1.25–7.69) | |
| Pre-stroke dependency* | 1.07 (0.69–1.66) | |
| Objective hemiparesis | 1.11 (0.60–2.06) | |
| High cholesterol† | 0.71 (0.09–5.46) | |
| NIHSS score | 1.07 (0.96–1.20) | |
| High systolic BP‡ | 1.01 (1.00–1.02) | |
| All-cause mortality | DWI-positive conversion | 9.04 (2.23–36.04) |
| Age | 1.10 (1.04–1.17) | |
| Male sex | 12.34 (1.32–115.38) | |
| Atrial fibrillation | 0.02 (0.00–1.34) | |
| Smoking | 1.10 (0.32–3.84) | |
| Pre-stroke dependency* | 1.83 (0.78–4.28) | |
| Objective hemiparesis | 0.44 (0.13–1.51) | |
| High cholesterol† | 4.71 (0.42–52.84) | |
| NIHSS score | 1.18 (0.99–1.40) | |
| High systolic BP‡ | 1.01 (0.99–1.03) | |
| Composite vascular outcome | DWI-positive conversion | 4.03 (2.16–7.51) |
| Age | 1.04 (1.02–1.06) | |
| Male sex | 1.29 (0.60–2.40) | |
| Atrial fibrillation | 0.49 (0.19–1.26) | |
| Smoking | 2.20 (1.04–4.61) | |
| Pre-stroke dependency* | 1.11 (0.76–1.62) | |
| Objective hemiparesis | 0.88 (0.50–1.54) | |
| High cholesterol† | 0.95 (0.22–4.17) | |
| NIHSS score | 1.11 (1.02–1.20) | |
| High systolic BP‡ | 0.99 (0.98–1.00) |
Calculated using Cox regression test for adjusted hazard ratio (adjusted for age, atrial fibrillation, smoking, pre-stroke dependency, objective hemiparesis, high cholesterol, NIHSS score, and high systolic BP).
OR, odds ratio; CI, confidence interval; DWI, diffusion-weighted imaging; NIHSS, National Institutes of Health Stroke Scale; BP, blood pressure.
Results
Table 3.
| Variable | Follow-up DWI-negative (n=377) | Follow-up positive-conversion (n=111) | P | OR on univariable analysis (95% CI) | OR on multivariable analysis (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Basic characteristics | |||||||
| Male sex | 219 (58.1) | 66 (59.5) | 0.80 | 1.06 (0.69–1.63) | – | ||
| Age at arrival (yr) | 62.8±13.5 | 62.3±13.4 | 0.72 | 1.00 (0.98–1.01) | – | ||
| Height (cm) | 163.4±8.5 | 163.8±8.4 | 0.88 | 1.00 (0.98–1.03) | 1.00 (0.96–1.05) | ||
| Weight (kg) | 65.2±11.2 | 64.9±11.8 | 0.61 | 1.00 (0.98–1.02) | 0.99 (0.97–1.02) | ||
| Body mass index (kg/m2) | 24.3±3.1 | 24.1±3.2 | 0.48 | 0.98 (0.91–1.05) | 0.98 (0.91–1.04) | ||
| Medical histories | |||||||
| Hypertension | 238 (63.1) | 73 (65.8) | 0.50 | 1.12 (0.72–1.76) | 1.18 (0.74–1.90) | ||
| Diabetic mellitus | 94 (24.9) | 25 (22.5) | 0.64 | 0.88 (0.52–1.43) | 0.89 (0.52–1.46) | ||
| Hyperlipidemia | 138 (36.6) | 40 (36.0) | 0.94 | 0.98 (0.62–1.51) | 0.98 (0.63–1.53) | ||
| Atrial fibrillation | 20 (5.3) | 26 (23.4) | <0.05 | 5.46 (2.92–10.35) | 6.17 (3.23–12.01) | ||
| Habitual smoking (current or ex-smoker) | 131 (34.7) | 64 (57.7) | <0.05 | 1.47 (1.15–1.88) | 3.76 (2.19–6.63) | ||
| Pre-stroke mRS* | 0 (0–0) | 0 (0–0) | <0.05 | 1.55 (1.11–2.15) | 1.62 (1.15–2.27) | ||
| 0 | 358 (95.0) | 98 (88.3) | – | – | – | ||
| 1 | 11 (2.9) | 5 (4.5) | – | – | – | ||
| ≥2 | 8 (2.1) | 8 (7.2) | – | – | – | ||
| Presenting neurologic deficits | |||||||
| Clear onset | 307 (81.4) | 85 (76.6) | 0.15 | 0.75 (0.45–1.26) | 0.67 (0.38–1.17) | ||
| Early neurologic deterioration | 4 (1.1) | 16 (14.4) | <0.05 | 15.71 (5.61–55.80) | 15.10 (5.71–47.66) | ||
| Dysarthria | 149 (39.5) | 61 (55.0) | 0.08 | 1.87 (1.22–2.87) | 1.52 (0.96–2.42) | ||
| Subjective hemiparesis | 196 (52.0) | 71 (64.0) | 0.05 | 1.64 (1.06–2.55) | 1.60 (1.00–2.58) | ||
| Objective hemiparesis | 11 (2.9) | 13 (11.7) | <0.05 | 4.41 (1.92–10.35) | 4.39 (1.90–10.32) | ||
| Objective arm weakness | 22 (5.8) | 14 (12.6) | 0.12 | 2.33 (1.13–4.68) | 1.85 (0.83–3.99) | ||
| Objective leg weakness | 29 (7.7) | 20 (18) | <0.05 | 2.64 (1.41–4.85) | 2.72 (1.38–5.30) | ||
| Dizziness | 79 (21.0) | 25 (22.5) | 0.60 | 1.10 (0.65–1.81) | 1.16 (0.66–2.00) | ||
| Veering tendency | 32 (8.5) | 10 (9.0) | 0.57 | 1.07 (0.48–2.17) | 1.26 (0.54–2.76) | ||
| Ataxia | 18 (4.8) | 5 (4.5) | 0.69 | 0.94 (0.30–2.42) | 0.80 (0.23–2.28) | ||
| Hypesthesia | 74 (19.6) | 25 (22.5) | 0.17 | 1.19 (0.70–1.97) | 1.48 (0.84–2.56) | ||
| Paresthesia | 43 (11.4) | 14 (12.6) | 0.53 | 1.12 (0.57–2.09) | 1.25 (0.60–2.48) | ||
| Paresthesia & hypesthesia, simultaneously | 15 (4.0) | 7 (6.3) | 0.08 | 1.62 (0.61–3.96) | 2.36 (0.85–6.04) | ||
| Aphasia | 21 (5.6) | 10 (9.0) | 0.19 | 1.68 (0.74–3.60) | 1.80 (0.72–4.20) | ||
| Diplopia | 14 (3.7) | 3 (2.7) | 0.36 | 0.72 (0.16–2.26) | 0.53 (0.11–1.83) | ||
| Duration of symptoms (min) | – | – | <0.05 | 1.36 (1.15–1.64) | 2.17 (1.57–3.08) | ||
| ≥60 | 167 (44.3) | 79 (71.2) | – | – | – | ||
| 10–59 | 94 (24.9) | 22 (19.8) | – | – | – | ||
| <10 or unknown | 116 (30.8) | 10 (9.0) | – | – | – | ||
| Time from the first symptom onset to the initial MR image (hr) | 4.6 (1.9–10.8) | 3.5 (1.9–9.8) | 0.63 | 1.00 (0.99–1.00) | 1.00 (0.99–1.07) | ||
| Time from the first symptom onset to follow-up MR image (hr) | 64.4 (49.4–86.6) | 68.5 (47.8–95.0) | 0.87 | 1.00 (1.00–1.01) | 1.00 (1.00–1.01) | ||
| Time from initial MR image to follow-up MR image (hr) | 54.0 (43.7–70.3) | 61.1 (42.5–81.1) | 0.48 | 1.01 (1.00–1.01) | 1.00 (0.99–1.01) | ||
| Initial NIHSS† | 0 (0–1) | 0 (0–2) | <0.05 | 1.60 (1.25–2.06) | 1.44 (1.08–1.91) | ||
| 0 | 262 (69.5) | 56 (50.5) | – | – | – | ||
| 1 | 53 (14.1) | 22 (19.8) | – | – | – | ||
| ≥2 | 62 (16.4) | 33 (29.7) | – | – | – | ||
| Laboratory abnormalities | |||||||
| Serum creatinine (mg/dL) | 0.88±0.57 | 0.87±0.34 | 0.96 | 0.99 (0.60–1.43) | 0.99 (0.58–1.45) | ||
| Hemoglobin (g/dL) | 13.9±1.8 | 14.1±1.5 | 0.35 | 1.06 (0.94–1.21) | 1.08 (0.93–1.26) | ||
| Total cholesterol level >240 mg/dL | 8 (2.1) | 10 (9.0) | <0.05 | 4.57 (1.76–12.25) | 4.70 (1.78–12.77) | ||
| Initial systolic BP per 1 mm Hg | 152.7±25.6 | 161.2±31.2 | <0.05 | 1.01 (1.00–1.02) | 1.01 (1.00–1.02) | ||
| Initial diastolic BP per 1 mm Hg | 85.2±15.7 | 88.0±18.9 | 0.15 | 1.01 (1.00–1.02) | 1.01 (1.00–1.02) | ||
Values are presented as number (%), mean±standard deviation, or median (interquartile range). Calculated by two-sample t-test for continuous variables or chi-square test for categorical variables.
DWI, diffusion-weighted imaging; OR, odds ratio; CI, confidence interval; mRS, modified Rankin Scale; MR, magnetic resonance; NIHSS, National Institutes of Health Stroke Scale; BP, blood pressure.
Table 4.
| Clinical factor | Points | β Regression coefficient (95% CI) |
|---|---|---|
| Total cholesterol level (mg/dL) | 1.27 (0.17 to 2.37) | |
| >240 | 4 | |
| ≤240 | 0 | |
| Blood pressure (mm Hg) | 0.36 (0.06 to 0.67) | |
| Systolic BP ≥180 or diastolic BP ≥110 | 2 | |
| 180> systolic BP ≥160 or 110> diastolic BP ≥100 | 1 | |
| Systolic BP<160 and diastolic BP<100, simultaneously | 0 | |
| Symptom duration (min) | 0.70 (0.35 to 1.05) | |
| ≥60 | 4 | |
| 10–59 | 2 | |
| 0–9 or unclear | 0 | |
| Atrial fibrillation | 1.67 (0.95 to 2.39) | |
| Yes | 6 | |
| No | 0 | |
| Habitual smoking | 0.83 (0.33 to 1.32) | |
| Current or ex-smoker | 3 | |
| Never smoker | 0 | |
| Objective motor power | 0.60 (–0.39 to 1.59) | |
| MRC grade of arm <5 and MRC grade of leg <5, simultaneously | 2 | |
| Other | 0 | |
| Aphasia | 0.79 (–0.14 to 1.72) | |
| Yes (any type of aphasia) | 3 | |
| No | 0 | |
| Dysarthria and subjective hemiparesis | 1.27 (0.72 to 1.82) | |
| Dysarthria and subjective hemiparesis, simultaneously | 4 | |
| Other | 0 | |
| Veering tendency and time from first symptom onset to initial MRI (hr) | 0.57 (–0.29 to 1.42) | |
| Veering tendency and <96, simultaneously | 2 | |
| Other | 0 | |
| Sensory | 1.03 (–0.02 to 2.09) | |
| Paresthesia and hypesthesia, simultaneously | 3 | |
| Other | 0 | |
| Pre-stroke mRS* | 0.52 (0.13 to 0.91) | |
| ≥2 | 6 | |
| 1 | 2 | |
| 0 | 0 |
Table 5.
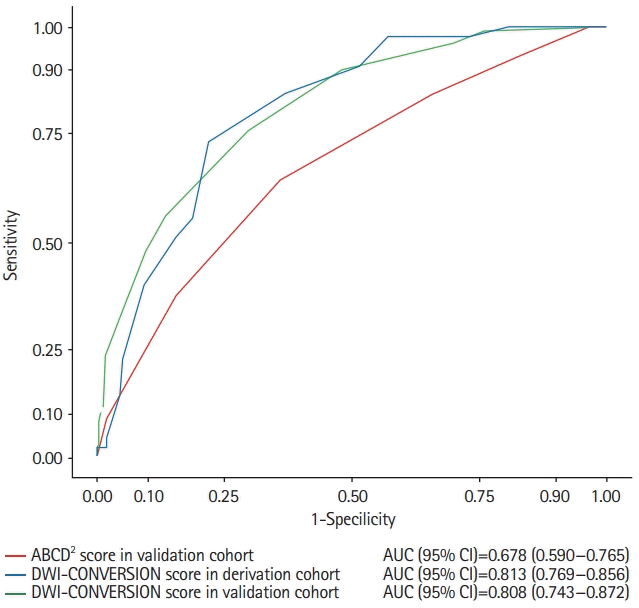
Figure 2.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download