Case Report
An 84-year-old man underwent laparoscopic low anterior resection and loop ileostomy for rectal cancer (pT2N0M0) after one month of preoperative chemotherapy and radiotherapy. Five months after the operation, he underwent endovascular aneurysmal repair of a right common iliac artery aneurysm. There was no prior history of diabetes mellitus or hypertension. On admission, complete blood cell counts and blood chemistry tests were within normal ranges except for the levels of blood urea nitrogen (BUN) (20.6 mg/dL; reference range 7.0–20.0 mg/dL) and creatinine (Cr) (1.25 mg/dL; reference range 0.6-1.2 mg/dL). After the fourth course of chemotherapy with leucovorin and 5-fluorouracil, the patient was scheduled for a colonoscopy. The bowel preparation was performed with 4 L of PEG-3350 solution after insertion of a Foley catheter into the distal ileum through the ileostomy for about 1hr.
However, only 200 mL of PEG-3350 fluid was expelled through the anus or ileostomy. One hour later, the patient complained of dyspnea and abdominal discomfort. When the rectal tube was inserted, about 300 mL of bloody fluid was drained. The patient’s vital signs were as follows: blood pressure, 170/100 mmHg; heart rate, 112 beats/min; respiratory rate, 38 breaths/minute; and blood oxygen saturation, 88% on room air. After oxygen was applied at 5 L/min by a simple mask, arterial blood gas analysis (ABGA) showed pH, 7.305; PaCO2, 32.7 mmHg; PaO2, 92.5 mmHg; HCO3 −, 15.8 mmol/L; base excess, −9.1; and O2 saturation, 95.7%.
Three hours later, the patient became drowsy and the systolic blood pressure decreased to 40 mmHg. The blood pressure increased to 90/60 mmHg after fluid resuscitation with 1 L normal saline, 500 mL colloid fluid and dopamine (20 μg/kg/min). The patient was transferred to the intensive care unit (ICU), and a ventilator was applied after intubation. Complete blood cell counts were normal. Blood chemistry tests showed glucose level, 217 mg/dL; BUN, 28.8 mg/dL; Cr, 1.93 mg/dL; sodium, 160 mEq/L; potassium, 6.1 mEq/L; and chloride, 115 mEq/L. Liver function was normal. ABGA showed pH, 6.858; PaCO
2, 48.1 mmHg; PaO
2, 119 mmHg; HCO
3 −, <10 mmol/L; base excess, −27.3; and O
2 saturation, 93.2%. The levels of the cardiac enzymes troponin-I and creatine kinase-MB were normal. The lactic acid level was 3.8 mmol/L and the albumin-corrected anion gap (AGc) was 43.25 mEq/L. On radiological examination there was hazy infiltration in both lower lung fields on the chest X-ray, and a large amount of ascites, only a very small amount of extraluminal air and pleural effusion on both sides on the abdominal computed tomography (
Fig. 1). Continuous fluid resuscitation, sodium bicarbonate infusion, vasopressor support and ventilator care were applied. During the emergency laparotomy, a large amount of bloody ascites thought to be leaked PEG-3350 solution were drained, and intra-mural hematoma with a bluish intestinal discoloration, severe intestinal wall edema and several serosal tears were found from the terminal ileum to the transverse colon, but the perforation site could not be founded because of the severe intestinal wall edema. A Foley catheter was inserted into the bowel lumen of the transverse colon through the ileostomy, and massive irrigation of the peritoneal cavity and drainage were performed.
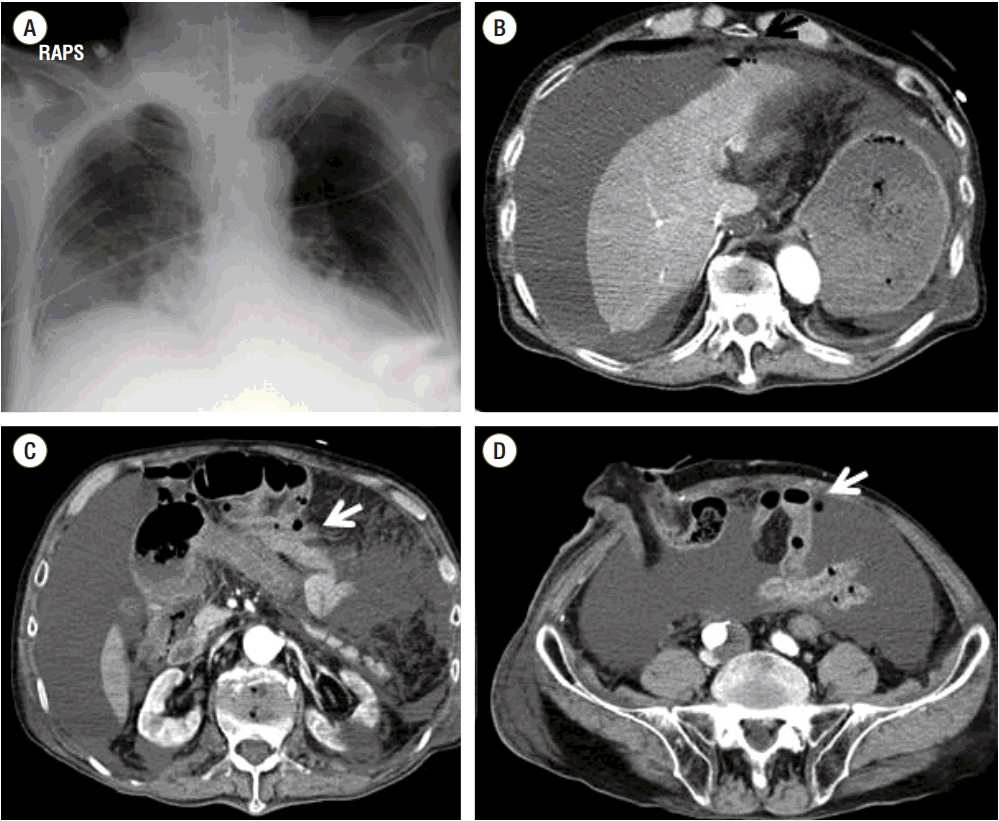 | Fig. 1.Chest X-ray (a) shows hazy infiltration in both lower lobe and abdominal computeco tomography (b, c, d) shows a large amount of ascites, a very small amount of extraluminal air (arrow) and pleural effusion on both sides. RAPS: right anterior posterior supine. 
|
When the patient returned to the ICU after the operation, vasopressor infusion with norepinephrine (0.3 μg/kg/min) and dopamine (11 μg/kg/min) was still needed to maintain the mean arterial blood pressure at > 65 mmHg. The postoperative AGc had decreased but was still high at 28 mEq/L, and lactic acid had decreased to 2.6 mmol/L. ABGA showed pH, 7.342; PaCO
2, 30.1 mmHg; PaO
2, 74.5 mmHg; HCO
3 −, 15.9 mmol/L; base excess, −8.3; and O
2 saturation, 94.3%. Sodium bicarbonate infusion was discontinued. Postoperative blood chemistry showed BUN, 30.5 mg/dL; Cr, 1.71 mg/dL; aspartate aminotransferase (AST), 45 U/L; alanine aminotransferase (ALT), 26 U/L; sodium, 156 mEq/L, potassium, 3.8 mEq/L; chloride, 116 mEq/L; and osmolality, 318 mOsm/kg. Fluid balance was positive 2600 mL on the operative day and positive 3100 mL on postoperative day 1. Central venous pressure was 7–8 cmH
2O. However, urine output decreased from 60 mL/h to 15 mL/h on postoperative day 1 and BUN, Cr and amylase increased to 46.6 mg/dL, 3.44 mg/dL and 2898 U/L, respectively. Sodium/potassium/chloride and osmolality were 151/4/107 mEq/L and 334 mOsm/kg, respectively. On ABGA, bicarbonate was down to 12.8 mEq/L, and AGc had increased to 35 mEq/L again (
Fig. 2). We suspected PEG poisoning on the basis of the high anion gap, hypernatremia and high osmolality, the relatively low lactic acid level and uncorrectable metabolic acidosis despite ample fluid resuscitation. Continuous renal replacement therapy (CRRT) was applied and the condition of the patient improved rapidly. Twenty-four hours after starting CRRT, ABGA showed pH, 7.414; PaCO
2, 37 mmHg; PaO
2, 60.8 mmHg; HCO
3 −, 23.2 mmol/L; base excess, −0.6; and O
2 saturation, 90.4%. Inotropics were discontinued 48 h after starting CRRT. Liver enzymes increased dramatically from ALT/AST 55/34 U/L to 1742/1399 U/L on postoperative day 1 and normalized on postoperative day 14. CRRT was stopped 5 days later. Extubation was performed on postoperative day 6, and the patient was transferred to the general ward. All of the microorganism cultures from the central blood, sputum, urine, rectal swab and nasal swab were negative. He discharged on postoperative day 19 and visited to outpatient clinic with tolerable condition after 2 weeks.
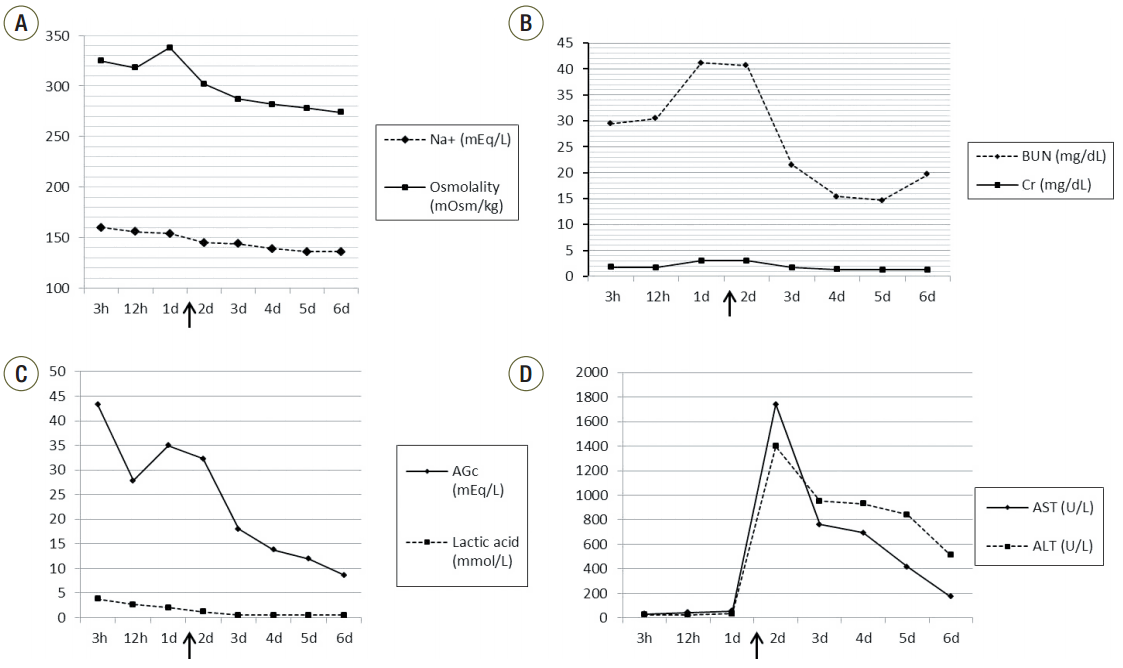 | Fig. 2.Graphs show the serial changes of serum sodium and osmolality (a), the blood urea nitrogen and creatinine (b), the albumin-corrected anion gap and lactic acid (c), and aspartate aminotransferase and alanine aminotransferase (d) over time after the infusion of PEG-3350. Arrows show the continuous renal replacement therapy starting points. Na+ : sodium; BUN: blood urea nitrogen; Cr: creatinine: AGc: albumin-corrected anion gap: AST: aspartate aminotransferase: ALT: alanine aminotransferase: CRRT: continuous renal replacement therapy; PEG: polyethylene glycol. 
|
Go to :

Discussion
PEG-3350 is poorly absorbed because of its large molecular weight, and thus can be used safely, even in elderly patients. Few published studies have assessed the absorption, distribution, metabolism and excretion of PEG. One study that performed PEG-3350 assays in humans reported that peak PEG-3350 plasma concentrations occurred at 2–4 h and usually declined to nonquantifiable levels within 18 h after single and multiple doses, with a half-life of about 4–6 h.[
2] The plasma peak concentration reached 500–600 ng/mL, and urinary excretion for 24 h had a mean of 28.8 mg (0.17%) following a single 17 g dose of PEG-3350. Mean fecal excretion of the administered dose was 93% in young subjects. Age, gender or mild kidney impairment did not alter the pharmacokinetics of PEG-3350. Another study evaluated the appearance of
14C PEG-4000 in intestinal venous blood after intraluminal administration into the jejunal, ileal and colonic segments. The absorption rate was low, especially in the colon. The amount absorbed into the blood during the collection period of 60 min was 1.4% in the jejunum and ileum, and 0.5% in the colon.[
3] A previous studies have demonstrated that PEG exerts more of an osmotic effect than would be predicted from its molecular weight. This osmotic effect may reflect interaction between PEG and water molecules, which may alter the physical chemistry of the solution and sequester water from the solution.[
4] Approximately three water molecules are bound to each ethylene glycol repeat unit in the polymer. This makes the effective molecular size of PEG even larger, restricting paracellular transport and making it much more difficult for PEG to permeate through lipid cell membranes than might otherwise be the case. There are strong interactions of PEG with mucus in the gastrointestinal tract.
All of these results were observed in the normal, intact gastrointestinal tract. However, when intestinal wall injury occurs and PEG-3350 solution leaks into the peritoneal cavity, the absorption, distribution and clearance of PEG-3350 will change, but there are no published data for humans. One study demonstrated that PEG-400 can have an irritant effect on serosal surfaces and causes subcapsular hepatocellular necrosis and low-grade peritonitis in mice when administered intraperitoneally at a high dose (4 mL/kg).[
5]
Despite our best efforts, we could not find any institution in Korea that was able to measure PEG in blood; therefore, the serum level of PEG could not be evaluated. However, in our case, the clinical findings such as hypotension, refractory metabolic acidosis with a high anion gap and the relatively low level of lactic acid despite ample fluid therapy, followed by acute renal failure, hepatic dysfunction and negative microbiology, may suggest the possibility of a correlation with PEG-3350 poisoning. Early CRRT appears to be a potentially useful treatment in such cases.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download