Abstract
Kawasaki disease (KD) is an acute systemic vasculitis of unknown etiology. We report a case of KD with acute respiratory distress syndrome (ARDS) after intravenous immunoglobulin (IVIG) infusion. Lung manifestations associated with KD have previously been reported in the literature. Although IVIG infusion is an effective therapy for acute KD, there are some reported complications related to IVIG infusion: hypotension, aseptic meningitis, acute renal failure, hemolytic anemia, etc. The case of KD reported here was treated with IVIG and aspirin. A few days after recovery from KD, the patient developed fever and maculopapular rash. A diagnosis of relapse KD was made and retreated with IVIG infusion. However, the patient developed ARDS four days after the second IVIG infusion. The patient recovered from ARDS after nine days of ICU care, which included high frequency oscillation ventilation with inhaled nitric oxide, steroid treatment and other supportive care.
Kawasaki disease (KD) is an acute inflammatory vasculitis of childhood, characterized by fever and signs of mucocutaneous inflammation.[1] The diagnosis of KD is based on a constellation of signs and symptoms, including fever, polymorphous exanthema, bilateral nonexudative conjunctivitis, inflammation of the lips and oral mucosa, changes in the extremities, and cervical adenopathy. As KD is an acute vasculitis, treatment should be tailored to reduce vascular inflammation. High-dose intravenous immunoglobulin (IVIG) and aspirin have been used to reduce inflammation. Patient with KD can also present with systemic symptoms not included in the diagnostic criteria, such as gastrointestinal complaints, jaundice, arthritis, arthralgia, aseptic meningitis, etc.[2] However, severe pulmonary manifestations are rare in KD.
Here, we report the case of a patient with relapse KD who unexpectedly developed acute respiratory distress syndrome (ARDS) after IVIG infusion.
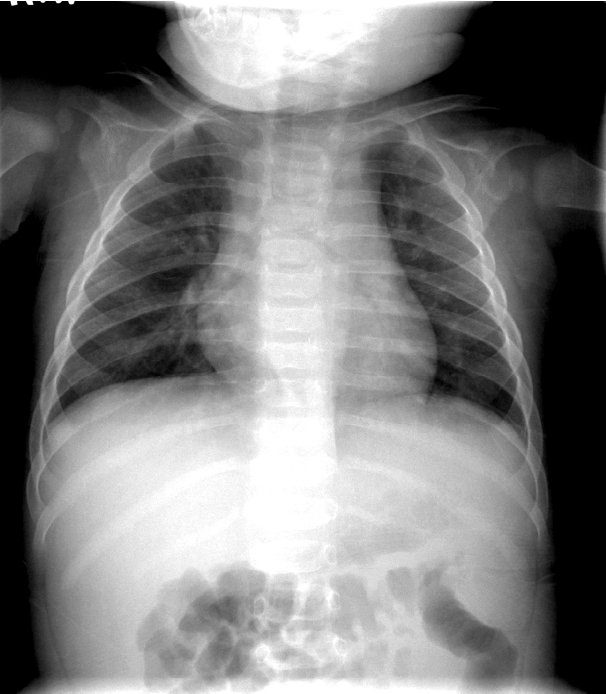
A 6-month-old boy presented to the emergency department with fever that continued for five days. In addition, he had developed polymorphous exanthema on the trunk, redness around the Bacillus Calmette-Guérin (BCG) vaccination site and lips, swelling of the hands and feet, and bilateral conjunctival injection. Chest radiography revealed normal findings on first admission (Fig. 1). After a diagnosis of typical KD was made, he was given IVIG and high-dose oral aspirin. He recovered from all symptoms related to KD within one week following his first admission and was discharged from our center with low-dose aspirin. Echocardiography, performed on the day of admission and before discharge, showed no complications of the coronary arteries.
However, on the sixth day after discharge, he revisited the emergency department with whole-body skin rash and fever that lasted for two days. The patient presented with an acute, unwell appearance, with reddish discoloration of his lips, bilateral conjunctival injection, and left cervical lymph node enlargement (about 1–1.5 cm). Maculopapular rash was noted on the face, trunk, and extremities, including the BCG site. The patient showed desquamation of the skin at the fingertips on the second admission. He exhibited a mild cough, sputum and rhinorrhea, without any abnormal findings on physical chest examination.
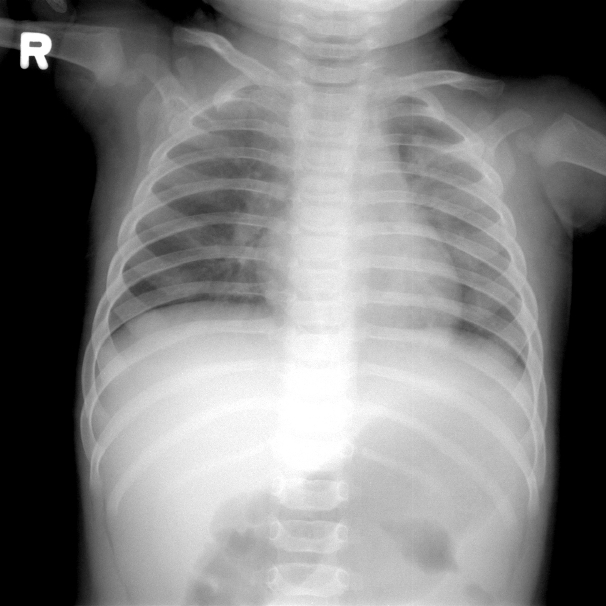
The laboratory findings were as follows: leukocyte count, 9,300/mm3; hemoglobin, 10.4 mg/dL; platelet count, 302,000/mm3; aspartate aminotransferase, 144 IU/L; alanine aminotransferase, 139 IU/L; erythrocyte sedimentation rate, 22 mm/h; and C-reactive protein, 0.17 mg/dL. Urinalysis revealed sterile pyuria. Chest radiography, on second admission, showed mild haziness in both lungs (Fig. 2). Echocardiography showed normal findings without dilatation of coronary arteries.
Under the diagnosis of relapse KD, a second dose of IVIG (2 g/kg) was administered. Fever subsided after the infusion of IVIG. However, on the third day after admission, the patient became lethargic, and blood and urine analysis was carried out. The laboratory findings were as follows: serum sodium, 116 mEq/L; serum osmolarity, 250 mOsm/L; urine sodium, 139 mEq/L; and urine osmolarity, 655 mOsm/L. In addition, serum levels of glucose, protein and triglyceride were normal, and the calculated osmolal gap was < 10 mOsm/kg. The syndrome of inappropriate antidiuretic hormone secretion was diagnosed and treated with 3% normal saline and furosemide. After two days, the serum Na+ level had increased to 137 mEq/L.
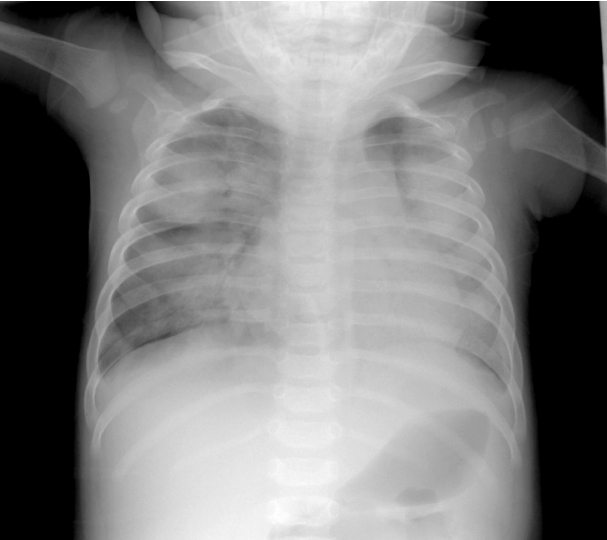
On the fourth day after IVIG infusion, the patient developed tachypnea, and O2 saturation suddenly decreased to 50% on room air, with the finding of multiple consolidations on chest radiography. The respiratory rate increased to 90/min. We detected rales on both whole lung fields and moderate chest retraction. Without CO2 retention on arterial blood gas analysis, the consolidations seen on radiographs rapidly became aggravated during the course of one day (Fig. 3). There was no evidence of pulmonary congestion. Hypoxemia worsened and the patient displayed drowsiness. He was transferred to the pediatric intensive care unit (PICU) and placed on a conventional ventilator after endotracheal intubation.
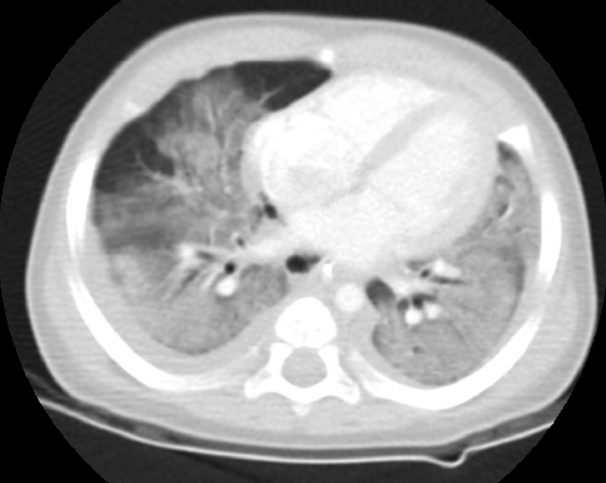
Upon arrival to the PICU, initial blood pressure was 63/39 mmHg and heart rate was 140 beats/min. The leukocyte count was 12,370/mm3, platelet count was 253,000/mm3, and C-reactive protein level was elevated to 10.39 mg/dL. The patient was treated with inotropic drugs and antibiotics (ampicillin/sulbactam and cefotaxime). Desaturation and hypercapnia were aggravated using a conventional ventilator, and therefore, the PICU team decided to ventilate the patient with a high frequency oscillatory ventilator (HFOV) and nitric oxide. To determine the cause of the acute respiratory distress, chest computed tomography (CT), echocardiography, and thoracentesis were carried out. Chest CT showed diffuse pulmonary ground-glass opacities and consolidation, with bilateral pleural effusion (Fig. 4). Echocardiography revealed normal heart function. Through thoracentesis, exudative fluid was obtained according to Light’s criteria and the results were as follows: WBC, >100/mL; RBC, 99/mL; polymorphonuclear leukocytes, 52%; lymphocytes, 10%; protein, 2,796 mg/dL; glucose, 139 mg/dL; lactate dehydrogenase, 26,963 U/L. Blood and respiratory cultures showed no bacterial growth. With a diagnosis of ARDS, intravenous hydrocortisone was administered daily.
On the fifth day after admission to the PICU, the patient was weaned from HFOV to the conventional mode of the ventilator. On the ninth day, the patient was taken off the ventilator. On the seventh day after transferring to the ward, he was discharged without lung, heart, or neurologic complications.
We have described a child with KD manifesting as ARDS, four days after a second dose of IVIG infusion. Although he showed normal findings on chest radiography on the first admission, chest radiography revealed mild haziness on the second admission, without respiratory symptoms or signs. The radiography findings on the second admission may be considered the acute phase of KD, according to a report in which approximately 15% of KD cases have abnormalities on chest radiography such as the presence of a reticulogranular pattern, peribronchial cuffing, atelectasis, air trapping, and pleural effusion.[3] Our patient developed ARDS after IVIG infusion on the second admission for recurrent KD. Our own assessment was that ARDS developed as late manifestation of relapse KD.
Pulmonary diseases have been reported in KD cases with most of them being mild, such as pneumonia and pulmonary nodules.[4,5] However, some authors reported that KD can manifest as a significant pulmonary dysfunction. Voynow et al.[6] described a 6-year-old girl with interstitial lung disease and pleural effusion associated with typical KD. In that case, the patient became hypoxic and received one dose of immunoglobulin and three pulses of solumedrol intravenously, resulting in resolution of her respiratory symptoms and signs. In a report by Palmer et al.[7] ARDS was seen in a child with KD. The response to two doses of IVIG was poor, with the patient remaining febrile and progressing to ARDS. After receiving another 2 g/kg dose of IVIG, he became afebrile and recovered. The authors assumed that ARDS probably resulted from a generalized capillary leak after a systemic inflammatory immune response. Yasukawa et al.[8] demonstrated that vascular endothelial growth factor and its receptors are increased in the blood vessels of KD patients and that this leads to perivascular edematous changes. Although those cases were similar to ours in respect of the progression to ARDS from KD, those cases developed ARDS during the active phase of KD, unlike our case, in which ARDS developed during the relapse of KD and after a second dose of IVIG. To the best of our knowledge, there have been no other reports of the occurrence of ARDS during the late phase of KD.
Considering the short interval between the first and second occurrence of KD, it might be thought that this patient had incompletely recovered from KD on the first admission. It is thought that unresolved KD might lead to capillary vascular permeability increase, resulting in ARDS. However, we should also consider that IVIG might also play a role in the progression to ARDS following KD, or complicate ARDS, considering the fact that ARDS developed after IVIG infusion, and not in the active phase of KD.
Gamma globulin is a biological product made from pooled donor plasma, and potentially important product-manufacturing differences exist. Perhaps for this reason, a few cases have reported that, as a blood component, IVIG infusion is associated with acute lung injury.[9–14] Transfusion-related acute lung injury (TRALI) typically presents within 1–6 hours after transfusion as a clinical syndrome characterized by severe respiratory distress, pulmonary edema, hypoxemia, normal left ventricular function, and fever.[15] There are two possible mechanisms that cause increased pulmonary vascular permeability, the hallmark of TRALI. The first, a single antibody-mediated event, involves transfusion of anti-neutrophil or anti-human leukocyte antigen antibodies. The second is the transfusion of substances such as cytokines and lipids that have neutrophil priming activity. TRALI after IVIG infusion has been reported in only six patients thus far.[9–14] Some of them were intubated with mechanical ventilation owing to severe hypoxemia. However, all of them were successfully extubated within a few days. Our case is not consistent with other cases of TRALI because of the long interval between the second dose of IVIG infusion and the onset of ARDS. Further studies should be carried out to delineate the possibility of late onset ARDS after IVIG.
Although the cause of ARDS after IVIG infusion in our case remains unanswered, we can consider several possibilities. Considering that activation of the immune system is a central feature of KD, some type of immune reaction may be involved in ARDS that stimulates an increase in vascular permeability, part of the ARDS pathophysiology.[16] Furthermore, we should consider that IVIG could boost the development of ARDS.
Prior to the development of ARDS, our case showed syndrome of inappropriate antidiuretic hormone on the third day following infusion of IVIG. Hyponatremia in KD has been reported since 1982.[17] Although the precise pathogenesis has yet to be clarified, it recently revealed that many inflammatory cytokines, active during the acute phase of KD, are related to antidiuretic hormone secretion. In addition, without specific management, hyponatremia normalizes in almost all cases. However, hyponatremia also developed in the subacute phase after IVIG infusion.[18] Such occurrences are often due to extrinsic factors such as fluid therapy. According to the literature, IVIG-related hyponatremia is considered to be a pseudohyponatremia, due to the osmotic load of the sugar-containing IVIG preparations. In our case, the laboratory findings revealed true hyponatremia, not pseudohyponatremia. Although hyponatremia developed during the late phase of KD in our case, it was more likely to be a complication of KD, rather than a complication of ARDS and IVIG infusion.
To summarize, we describe a case of ARDS associated with late phase KD. We could not differentiate between KD itself and IVIG complications as the exact cause of ARDS. Severe pulmonary manifestation is rare in KD and no adequate reference cases were available for comparison. The relationship between ARDS and KD remains unclear, and further studies of greater numbers of cases with similar symptoms are needed. Clinicians monitoring patients with KD should be cautious when respiratory signs or symptoms are seen. If patients show symptoms related to acute lung injury, administration of intravenous steroids should be considered to prevent progression to severe ARDS.
References
1). Bayers S, Shulman ST, Paller AS. Kawasaki disease: part I. Diagnosis, clinical features, and pathogenesis. J Am Acad Dermatol. 2013; 69:501.
2). Sánchez-Manubens J, Bou R, Anton J. Diagnosis and classification of Kawasaki disease. J Autoimmun. 2014; 48–49:113–7.

3). Umezawa T, Saji T, Matsuo N, Odagiri K. Chest x-ray findings in the acute phase of Kawasaki disease. Pediatr Radiol. 1989; 20:48–51.

4). Uziel Y, Hashkes PJ, Kassem E, Gottesman G, Wolach B. “Unresolving pneumonia” as the main manifestation of atypical Kawasaki disease. Arch Dis Child. 2003; 88:940–2.

5). Freeman AF, Crawford SE, Finn LS, López-Andreu JA, Ferrando-Monleón S, Pérez-Tamarit D, et al. Inflammatory pulmonary nodules in Kawasaki disease. Pediatr Pulmonol. 2003; 36:102–6.

6). Voynow JA, Schanberg L, Sporn T, Kredich D. Pulmonary complications associated with Kawasaki disease. J Pediatr. 2002; 140:786–7.

7). Palmer AL, Walker T, Smith JC. Acute respiratory distress syndrome in a child with Kawasaki disease. South Med J. 2005; 98:1031–3.

8). Yasukawa K, Terai M, Shulman ST, Toyozaki T, Yajima S, Kohno Y, et al. Systemic production of vascular endothelial growth factor and fms-like tyrosine kinase-1 receptor in acute Kawasaki disease. Circulation. 2002; 105:766–9.
9). Rizk A, Gorson KC, Kenney L, Weinstein R. Transfusion-related acute lung injury after the infusion of IVIG. Transfusion. 2001; 41:264–8.

10). Dooren MC, Ouwehand WH, Verhoeven AJ, von dem Borne AE, Kuijpers RW. Adult respiratory distress syndrome after experimental intravenous gamma-globulin concentrate and monocyte-reactive IgG antibodies. Lancet. 1998; 352:1601–2.
11). Berger-Achituv S, Ellis MH, Curtis BR, Wolach B. Transfusion-related acute lung injury following intravenous anti-D administration in an adolescent. Am J Hematol. 2008; 83:676–8.

12). Voulgari PV, Paschou S, Svarna E, Tsifetaki N, Drosos AA. Images in rheumatology. Transfusion-related acute lung injury during intravenous immunoglobulin treatment. J Rheumatol. 2010; 37:190–1.
13). Gupta V, Gupta P, Yadav TP. Transfusion related acute lung injury with intravenous immunoglobulin. Indian Pediatr. 2011; 48:807–8.
14). Stoclin A, Delbos F, Dauriat G, Brugière O, Boeri N, Métivier AC, et al. Transfusion-related acute lung injury after intravenous immunoglobulin treaetment in a lung transplant recipient. Vox Sang. 2013; 104:175–8.
15). Kleinman S, Caulfield T, Chan P, Davenport R, McFarland J, McPhedran S, et al. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004; 44:1774–89.

16). Pierrakos C, Karanikolas M, Scolletta S, Karamouzos V, Velissaris D. Acute respiratory distress syndrome: pathophysiology and therapeutic options. J Clin Med Res. 2012; 4:7–16.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download