Abstract
Background
The role of extended-spectrum β-lactamase (ESBL)-producing or multidrug-resistant (MDR) organisms in patients with sepsis secondary to urinary traction infection (UTI) has not been investigated extensively in the intensive care unit (ICU) setting.
Methods
Patients with UTI sepsis admitted to the ICU were retrospectively enrolled in this study (January 2009–December 2012). We investigated the impact of ESBL-producing and ESBL-negative MDR organisms on hospital outcome.
Results
In total, 94 patients were enrolled (median age, 73.0 years; female, 81.9%), and ESBL-producing and ESBL-negative MDR organisms accounted for 20.2% (n = 19) and 30.9% (n = 29), respectively. Both patients with ESBL-producing and ESBL-negative MDR organisms were more likely to experience a delay in adequate antibiotic therapy than those with non-ESBL/non-MDR organisms (p < 0.001 and p = 0.032, respectively). However, only patients with ESBL-producing organisms showed a higher mortality rate (ESBL vs. ESBL-negative MDR vs. non-ESBL/non-MDR, 31.6% vs. 10.3%.vs. 10.9%, respectively). In multivariate analyses, ESBL production was significantly associated with hospital mortality (odds ratio, 11.547; 95% confidence interval, 1.047–127.373), and prior admission was a significant predictor of ESBL production.
Urinary tract infection (UTI) is one of the most common infectious diseases in both community and hospital settings and may be responsible for 20–30% of all cases of sepsis.[1,2] The frequency of infections by drug-resistant pathogens is increasing, and some patient groups with UTIs still have high mortality rates.[3]
Multidrug resistance (MDR) is an increasing concern worldwide, and extended-spectrum β-lactamase (ESBL) is one of the most important plasmid-mediated β-lactamases produced by drug-resistant enterobacteria. Recently, the prevalence of ESBL producers in UTIs caused by Escherichia coli and Klebsiella pneumoniae was reported to be > 50% in several Asian countries.[4,5] Although MDR and ESBL productions are associated with a delay in the administration of appropriate antibiotics in patients with UTI or bacteremia, data on their associations with hospital mortality are conflicting.[6–9] However, the severity of illness is usually high, and early administration of appropriate antibiotics is important for patients with sepsis admitted to the intensive care unit (ICU). Additionally, antibiotic treatment options for MDR or ESBL-producing organisms are limited. Therefore, although mortality is relatively lower in patients with urinary-source compared with non-urinary-source sepsis,[10,11] UTI sepsis caused by MDR organisms may be important among patients admitted to the ICU.
To date, many authors have investigated the prevalence of and risk factors for MDR or ESBL in patients with UTI.[6,7,12,13] However, data on the impact of ESBL or ESBL-negative MDR on hospital outcome in the ICU setting are limited. Therefore, in this study, we investigated the impact of ESBL or ESBL-negative MDR on hospital mortality in UTI sepsis patients admitted to the ICU.
This was a retrospective cohort study conducted in a 16-bed medical ICU at Hallym University Sacred Heart Hospital (an 800-bed tertiary university hospital) between January 2009 and December 2012. Adult patients (≥ 18 years) diagnosed with UTI sepsis and admitted to the ICU were included in the study. The following were used as exclusion criteria: no organisms identified or polymicrobial infection, no outcome data due to transfer to another hospital, do-not-resuscitate status or treatment restrictions, cardiopulmonary resuscitation at an emergency department or ICU admission, or an immunocompromised status (e.g., inherited genetic diseases, acquired diseases [e.g., HIV, solid or hematologic cancer], and medications [e.g., steroid, chemotherapy, radiotherapy, other immunosuppressant agents]). However, patients were eligible when their cancers had been in complete remission for > 6 months or when they were being treated with a low-dose steroid (i.e., ≤ 10 mg/day prednisolone or equivalent).
We collected the following data: age, gender, prior history, comorbid illnesses, Charlson comorbidity index, and Simplified Acute Physiology Score (SAPS) II at ICU admission. Laboratory data, including routine chemistry and cardiac markers, were also collected. We evaluated the inappropriateness of empirical antibiotics and measured time intervals (i.e., hour) from ICU admission to the treatment with appropriate antibiotics for all patients that were enrolled. We investigated the ICU and hospital mortality rates, as well as the length of hospital stay (i.e., from ICU admission to hospital discharge). The Institutional Review Board at Hallym University Sacred Heart Hospital approved this study (IRB No. 2014-I134), and the requirement for informed consent was waived due to the retrospective nature of the study.
UTI sepsis was defined by the International Sepsis Forum Consensus Conference Definitions of Infection in the ICU.[11,14] For noncatheterized patients, at least two of the following criteria were required: fever (> 38ºC); urgency; localized pain or tenderness at the involved site; and any of the following: pyuria, bacteriuria, purulent discharge from the affected site, hematuria, positive Gram stain, organism isolated from urine culture (> 105 colony-forming units [CFU]/ml), or radiologic evidence of infection (e.g., hydronephrosis, stenosis, renal abscess, etc). Catheterized patients were required to meet one of the following criteria: localizing signs or symptoms compatible with UTI, a positive blood culture, or imaging that supported a urinary source, in addition to a positive urine culture (> 105 CFU/ml).
Sepsis was defined as the probable or documented presence of infection in addition to systemic manifestations of infection. Severe sepsis was defined as sepsis plus sepsis-induced organ dysfunction or tissue hypoperfusion. Septic shock was defined as severe sepsis with hypotension despite adequate fluid resuscitation.[15] Community-acquired infection was defined as a positive urine culture obtained at the time of hospital admission or < 48 hours after hospitalization; however, an infection was classified as healthcare-associated if a patient met any of the healthcare-risk factors as reported by Friedman et al.[16] Hospital-acquired infections were defined as a positive culture obtained > 48 hours after hospitalization.
Species identification and antimicrobial susceptibility tests were performed using the MicroScan Neg Breakpoint Combo Panel Type 42 (Siemens Healthcare Diagnostics, Inc., West Sacramento, CA, USA) in accordance with the manufacturer’s instructions. The production of ESBL was determined by a double-disk synergy test as described by the Clinical and Laboratory Standards Institute.[17] A pathogen was defined as MDR when it was resistant to at least three classes of antibiotics.[13]
In terms of antibiotic treatments, patients were treated empirically according to the treatment guidelines at ICU admission.[14,18] However, as soon as the causal pathogen was identified, antibiotics were adjusted according to the susceptibility test results. Empirical antibiotics were considered appropriate when isolated organisms were susceptible to the antibiotics.
The primary outcome was the relationship between ESBL-producing or ESBL-negative MDR organisms and hospital mortality in patients with UTI sepsis in the ICU setting. The secondary outcomes were the initial rates of inappropriate antibiotic administration for ESBL-producing or ESBL-negative MDR organisms and several risk factors for ESBL production in patients with UTI sepsis.
Data are presented as median (interquartile range, IQRs) for continuous data and percentage for categorical data. For comparisons between two groups, the Mann-Whitney U test was used for continuous data, while the chi-square or Fisher’s exact test was used for categorical data. Multivariate logistic regression analyses were performed using covariates with p < 0.05 in the univariate analyses. The Kaplan-Meier method with the log-rank test was also performed. All reported p values were two-sided, and p < 0.05 was deemed to indicate statistical significance. All analyses were conducted using the SPSS statistical software (IBM SPSS Statistics version 21, Standard for Medical Network, Armonk, NY, USA).
In total, 125 patients with UTI sepsis, severe sepsis, or septic shock were initially screened. Of these, 94 were enrolled in the present study (Fig. 1). The mean age was 73.0 years (65.0–81.0 years), and 81.9% of the patients were female (Table 1). Fifty-five patients (58.5%) had community-acquired infections, 25 patients had healthcare-associated infections, and 14 had hospital-acquired infections. Forty-nine patients (52.1%) were bed-ridden, and 19 (20.2%) had a history of prior UTI within the last year. Thirty patients (31.9%) had structural abnormalities or lesions in the urinary tract. Eight patients (8.5%) underwent percutaneous nephrostomy, 65 (69.1%) received vasopressors, and 29 (30.9%) were treated with mechanical ventilation.
Among the causal organisms, E. coli was the most common pathogen (n = 66, 70.2%); five patients had Gram-positive infections, and one patient had a fungal infection (Table 2). ESBL-producing and ESBL-negative MDR organisms accounted for 20.2% (n = 19) and 30.9% (n = 29), respectively, and one patient had a carbapenem-resistant organism. Among the E. coli isolates, 21.2% were ESBL producers and 31.8% were ESBL-negative MDR organisms. The ESBL rates of Proteus mirabilis and K. pneumoniae were 42.9% (3/7) and 33.3% (2/6), respectively. The rate of bacteremia was 63.2%, 72.4%, and 69.6% in patients with ESBL-producing, ESBL-negative MDR, and non-ESBL/non-MDR organisms, respectively (p = 0.791).
In this study, β-lactams (n = 27) and quinolones (n = 28) were the most commonly used agents for empirical treatments (carbapenems, n = 22; β-lactam/β-lactamase inhibitors, n = 10; β-lactam/β-lactamase inhibitor and quinolone combinations, n = 6; other combinations, n = 1), and 16.0% (15/94) of all enrolled patients initially received inappropriate antibiotics. However, the rates of initial inappropriate antibiotic administration were higher in both patients with ESBL-producing and ESBL-negative MDR organisms compared with cases with non-ESBL/non-MDR organisms (42.1 % vs. 20.7% vs. 2.2%, respectively; Fig. 2). The time to appropriate antibiotic treatment was also longer in both of the MDR groups (p < 0.001 and p = 0.033; Fig. 3). However, the type of infection (i.e., community-acquired vs. health-care-associated vs. hospital-acquired infection) was not associated with antibiotic inappropriateness (16.4% vs. 12.0% vs. 21.4%, p = 0.737)
ICU and hospital mortality rates were 12.8% (12/94) and 14.9% (14/94), respectively. Patients with ESBL producers showed a higher mortality rate than the other two groups (31.6% vs. 10.3% [ESBL-negative MDR organisms] vs. 10.9% [non-ESBL/non-MDR organisms]; Fig. 4); there was a significant difference between patients with ESBL producers and other groups (p = 0.033). Among patients with E. coli isolates, the hospital mortality was 28.6% and 14.3% in those with ESBL-positive and ESBL-negative MDR isolates, respectively. However, the type of infection did not have an impact on hospital mortality (10.9% vs. 20.0% vs. 21.4% in community-acquired vs. healthcare-associated vs. hospital-acquired infection, respectively, p = 0.433). The length of hospital stay tended to be greater in patients with ESBL producers than in patients without ESBL producers (14.0 days [13.0–22.0 days] vs. 12.0 days [9.0–19.0 days], p = 0.061).
In univariate analyses, Charlson comorbidity index and comorbid illnesses were not associated with hospital mortality. However, hematocrit, brain natriuretic peptide (BNP), SAPS II, ESBL production, and inappropriate antibiotics were significant factors (Table 3). These five variables were included into the multivariate analysis (Hosmer-Lemeshow goodness-of-fit test, chi-square = 1.187, and p = 0.997; Table 3), which revealed that ESBL production was significantly associated with increased hospital mortality (odds ratio [OR], 11.547; 95% confidence interval [CI], 1.047–127.373); when the age variable was added to the model, the OR of ESBL production was 13.938 (95% CI, 1.074–180.831). However, when the variable of inappropriate antibiotic administration was excluded from the model, the OR of ESBL production increased to 21.971 (95% CI, 2.335–206.741).
Among the clinical and laboratory variables, prior admission within one year (p = 0.005), hospital-acquired infection (p = 0.007), and a bed-ridden state (p = 0.009) were more frequently identified in ESBL-positive patients than in ESBL-negative patients. In the multivariate model (Hosmer-Lemeshow goodness-of-fit test, chi-square = 3.119, and p = 0.682), prior admission within one year (p = 0.032) and hospital-acquired infection (p = 0.047) were significantly associated with ESBL production; however, when the age variable was added to the model, only prior admission within one year (p = 0.032) was a significant risk factor.
This study had several findings; first, ESBL-producing and ESBL-negative MDR organisms accounted for 20.2% and 33.9%, respectively, of infections in patients admitted to the ICU for UTI sepsis. Second, both ESBL production and ESBL-negative MDR were associated with a delay in the administration of appropriate antibiotic treatment; however, only ESBL production, not ESBL-negative MDR, was an independent risk factor for hospital mortality in patients with UTI sepsis. Third, a history of hospital admission was a significant risk factor for ESBL production.
There has been an increase in the emergence of drug-resistant organisms in the past two decades, and many authors have studied the incidence and risk factors of ESBL production in patients with UTI.[7,19–21] In previous studies, several authors reported that ESBL production or MDR is associated with inappropriate antibiotic administration or an increased length of hospital stay. However, UTI is known to be associated with a low risk of mortality among patients with septicemia,[11] and the relationships between MDR organisms and increased mortality rates remain debatable.[9,19,22,23] However, because treatment options are limited in MDR infections, these infections could have a significant impact on hospital outcomes in critically ill patients, especially in the ICU setting.
Worldwide, 17.9% of E. coli isolates from UTI specimens are ESBL producers, and the Asia-Pacific region has the highest ESBL rate (i.e., 27.7%).[24] According to the Study for Monitoring Antimicrobial Resistance Trends (SMART) study by Lu et al.[4] China and Vietnam have the highest ESBL rates of 60%, and Singapore, Malaysia, and Thailand have rates of ∼30% among isolates that cause UTI. In the present study, we found an ESBL rate of 20.2% among all isolates and 21.2% among E. coli isolates; these rates are similar to the Korean data from the SMART study. However, the ESBL rate among patients with E. coli bacteremia was higher (17.4%, 8/46) than those in previous studies.[8,20] In particular, P. mirabilis (42.9%) and K. pneumoniae (33.3%) had higher rates of ESBL production than did E. coli isolates. The ESBL rate among K. pneumoniae isolates was also higher (26%), as reported in a Taiwanese nationwide surveillance study.[25] The authors noted an increase in the rate of ESBL production among K. pneumoniae isolates in their ICUs. Therefore, continuous surveillance for these ESBL-producing organisms is needed among patients admitted to the ICU for UTI sepsis.
Many risk factors associated with ESBL production have been identified in previous studies,[9,12,19] including previous antibiotic use (especially fluoroquinolones), previous admission, male gender, diabetes mellitus, and recurrent UTI. In this study, we also found that prior admission within one year was a risk factor. However, some studies have indicated that inpatient infection or complicated UTI is more likely to be associated with ESBL production than is outpatient infection or uncomplicated UTI.[26,27] Therefore, healthcare practitioners should be attuned to the possibility of ESBL production in patients with UTI sepsis and/or hospital-acquired infection.
Infections caused by ESBL or MDR organisms are known to be associated with increased morbidity and mortality,[28,29] which is primarily associated with a delay in adequate antibiotic therapy. Although we observed a higher mortality in patients with inappropriate antibiotics compared to those with appropriate antibiotics, there was no significant association in the multivariate analysis in this study. Several previous studies have shown conflicting results.[6,8,22,30] However, a significant number of elderly patients with severe illness (i.e., SAPS II) were enrolled in the present study and could have died due to underlying disease or severe illness rather than to the actual infection.
With regard to patient outcome, we reported a hospital mortality of 14.9% in the present study. Although this result is higher than those of other studies,[31,32] patient age, comorbidity, and severity of illness at ICU admission must also be considered. The previous mortality rate for patients with septic shock due to a urinary source ranged from 10% to 20%, which was lower than the rates for other common septic shock sources.[11] Other Korean studies also reported mortality rates in the range of 9.1%–25.9%.[11] In particular, Chang et al.[33] reported that E. coli had a lower case fatality rate than all other Gram-negative infections among patients with hospital-acquired UTI. This low mortality rate is considered to be associated with the relatively straightforward treatment for UTI. However, considering the importance of appropriate empirical treatment for severe MDR pathogenic infections, physicians should consider individual risk factors, clinical severity, and local epidemiology when making decisions about patient treatment.
Many authors have discussed ESBL production rather than MDR when studying patients with UTI. Although all ESBL producers were MDR organisms in our study, we found that only ESBL production, not ESBL-negative MDR, was significantly associated with increased mortality in the multivariate analysis. This suggests that the important prognostic factor among patients with UTI sepsis admitted to the ICU is not whether the causal organism is MDR, but rather, whether the organism is an ESBL producer. In previous studies, Menashe et al.[34] and Kim et al.[35] determined that there was no significant association between ESBL production and mortality. However, in a meta-analysis by Rottier et al.,[28] the authors reported that bacteremia caused by an ESBL producer was associated with a higher mortality than bacteremia with ESBL-negative organisms, and the higher mortality was likely to be mediated through inappropriate antibiotic therapy. However, we also found that adjusting for inappropriate antibiotic treatments decreased the OR of ESBL production, which was indicated in the study by Rottier et al. Therefore, in our study, the effect of ESBL production on hospital mortality can partially be explained by inappropriate antibiotic treatments. Additionally, we found that BNP was also significantly associated with hospital mortality. Recent studies suggested that BNP may be a powerful predictor of mortality in patients with sepsis.[36,37] Therefore, taking this factor into consideration might also provide a better prognosis for patients with UTI sepsis admitted to the ICU.
There were several limitations to this study. First, the 95% CIs for ESBL producers in the multivariate analysis were wide because the sample size was small. Second, there is a possibility of unintended bias due to the retrospective nature of the study. Third, the ESBL-negative MDR organisms were heterogeneous and may have different antibiotic resistance mechanisms. Therefore, these limitations should be considered when the results are interpreted. However, despite these limitations, our study had several major strengths. First, we collected an extensive range of patient data, including the status of indwelling urinary catheters, structural lesions in the urinary tract, and prior history of UTI. In addition, we focused only on septic patients with a severe illness diagnosis who had been admitted to the ICU.
This study determined that, although both ESBL-producing and ESBL-negative MDR organisms are associated with a delay in appropriate empirical treatments, only ESBL production, not ESBL-negative MDR, is a significant predictor of hospital mortality. Therefore, it is necessary to evaluate the possibility of ESBL-producing organisms when deciding on empirical antibiotic treatment, and ESBL production should be considered when diagnosing patients with UTI sepsis in the ICU setting.
Acknowledgments
The authors thank resident physicians and registered nurses in the ICU for their dedication to this study.
References
1). Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003; 348:1546–54.

2). Brun-Buisson C, Meshaka P, Pinton P, Vallet B; EPISEPSIS Study Group. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Med. 2004; 30:580–8.
3). Rosser CJ, Bare RL, Meredith JW. Urinary tract infections in the critically ill patient with a urinary catheter. Am J Surg. 1999; 177:287–90.

4). Lu PL, Liu YC, Toh HS, Lee YL, Liu YM, Ho CM, et al. Epidemiology and antimicrobial susceptibility profiles of gram-negative bacteria causing urinary tract infections in the Asia-Pacific region: 2009–2010 results from the study for monitoring antimicrobial resistance trends (SMART). Int J Antimicrob Agents. 2012; 40:S37–43.

5). Hawser SP, Bouchillon SK, Hoban DJ, Badal RE, Hsueh PR, Paterson DL. Emergence of high levels of extended-spectrum-beta-lactamase-producing gram-negative bacilli in the Asia-Pacific region: data from the study for monitoring antimicrobial resistance trends (SMART) program, 2007. Antimicrob Agents Chemother. 2009; 53:3280–4.
6). Frakking FN, Rottier WC, Dorigo-Zetsma JW, van Hattem JM, van Hees BC, Kluytmans JA, et al. Appropriateness of empirical treatment and outcome in bacteremia caused by extended-spectrum-β -lactamase-producing bacteria. Antimicrob Agents Chemother. 2013; 57:3092–9.
7). Lin HC, Lai LA, Wu JY, Su YM, Chang SP, Hsueh YM. Risk factors for acquiring extended-spectrum β -lactamase-producing Enterobacteriaceae in geriatric patients with multiple comorbidities in respiratory care wards. Geriatr Gerontol Int. 2013; 13:663–71.
8). Park SH, Choi SM, Lee DG, Kim J, Choi JH, Kim SH, et al. Emergence of extended-spectrum β -lactamase-producing escherichia coli as a cause of community-onset bacteremia in South Korea: risk factors and clinical outcomes. Microb Drug Resist. 2011; 17:537–44.
9). Rodríguez-Baño J, Picón E, Gijón P, Hernández JR, Ruíz M, Peña C, et al. Community-onset bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli: risk factors and prognosis. Clin Infect Dis. 2010; 50:40–8.
10). Ku NS, Kim YC, Kim MH, Song JE, Oh DH, Ahn JY, et al. Risk factors for 28-day mortality in elderly patients with extended-spectrum β -lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae bacteremia. Arch Gerontol Geriatr. 2014; 58:105–9.
12). Hayakawa K, Gattu S, Marchaim D, Bhargava A, Palla M, Alshabani K, et al. Epidemiology and risk factors for isolation of Escherichia coli producing CTX-M-type extended-spectrum β -lactamase in a large U.S. medical center. Antimicrob Agents Chemother. 2013; 57:4010–8.
13). Linhares I, Raposo T, Rodrigues A, Almeida A. Frequency and antimicrobial resistance patterns of bacteria implicated in community urinary tract infections: a ten-year surveillance study (2000–2009). BMC Infect Dis. 2013; 13:19.

14). Calandra T, Cohen J. International Sepsis Forum Definition of Infection in the ICU Consensus Conference: The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med. 2005; 33:1538–48.
15). Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013; 39:165–228.

16). Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, et al. Healthcare--associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002; 137:791–7.
17). Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: seventeenth informational supplement M100-S17. Wayne, PA: CLSI;2007.
18). The Korean Society of Infectious Diseases; The Korean Society for Chemotherapy; Korean Association of Urogenital Tract Infection and Inflammation; The Korean Society of Clinical Microbiology. Clinical guideline for the diagnosis and treatment of urinary tract infections: asymptomatic bacteriuria, uncomplicated & complicated urinary tract infections, bacterial prostatitis. Infect Chemother. 2011; 43:1–25.
19). Briongos-Figuero LS, Gómez-Traveso T, Bachiller-Luque P, Domínguez-Gil González M, Gómez-Nieto A, Palacios-Martín T, et al. Epidemiology, risk factors and comorbidity for urinary tract infections caused by extended-spectrum beta-lactamase (ESBL)-producing enterobacteria. Int J Clin Pract. 2012; 66:891–6.

20). Sader HS, Flamm RK, Jones RN. Frequency of occurrence and antimicrobial susceptibility of gram-negative bacteremia isolates in patients with urinary tract infection: results from United States and European hospitals (2009–2011). J Chemother. 2014; 26:133–8.

21). Zilberberg MD, Shorr AF. Secular trends in gram-negative resistance among urinary tract infection hospitalizations in the United States, 2000–2009. Infect Control Hosp Epidemiol. 2013; 34:940–6.

22). Lin JN, Chen YH, Chang LL, Lai CH, Lin HL, Lin HH. Clinical characteristics and outcomes of patients with extended-spectrum β -lactamase-producing bacteremias in the emergency department. Intern Emerg Med. 2011; 6:547–55.
23). Lye DC, Earnest A, Ling ML, Lee TE, Yong HC, Fisher DA, et al. The impact of multidrug resistance in healthcare-associated and nosocomial gram-negative bacteraemia on mortality and length of stay: cohort study. Clin Microbiol Infect. 2012; 18:502–8.

24). Hoban DJ, Nicolle LE, Hawser S, Bouchillon S, Badal R. Antimicrobial susceptibility of global inpatient urinary tract isolates of Escherichia coli: results from the study for monitoring antimicrobial resistance trends (SMART) program: 2009–2010. Diagn Microbiol Infect Dis. 2011; 70:507–11.

25). Jean SS, Hsueh PR, Lee WS, Chang HT, Chou MY, Chen IS, et al. Nationwide surveillance of antimicrobial resistance among Enterobacteriaceae in intensive care units in Taiwan. Eur J Clin Microbiol Infect Dis. 2009; 28:215–20.

26). Azap OK, Arslan H, Serefhanoğlu K, Colakoğlu S, Erdoğan H, Timurkaynak F, et al. Risk factors for extended-spectrum beta-lactamase positivity in uropathogenic Escherichia coli isolated from community-acquired urinary tract infections. Clin Microbiol Infect. 2010; 16:147–51.
27). Lee DS, Lee CB, Lee SJ. Prevalence and risk factors for extended spectrum beta-lactamase-producing uropathogens in patients with urinary tract infection. Korean J Urol. 2010; 51:492–7.

28). Rottier WC, Ammerlaan HS, Bonten MJ. Effects of confounders and intermediates on the association of bacteraemia caused by extended-spectrum β -lactamase-producing Enterobacteriaceae and patient outcome: a meta-analysis. J Antimicrob Chemother. 2012; 67:1311–20.
29). Rodríguez-Baño J, Pascual A. Clinical significance of extended-spectrum beta-lactamases. Expert Rev Anti Infect Ther. 2008; 6:671–83.
30). Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, et al. Bloodstream infections due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for mortality and treatment outcome, with special emphasis on antimicrobial therapy. Antimicrob Agents Chemother. 2004; 48:4574–81.
31). Kang CI, Kim SH, Bang JW, Kim HB, Park SW, Choe YJ, et al. Risk factors for infection and treatment outcome of bloodstream infections due to extended spectrum beta-lactamases producing Klebsiella pneumoniae. Infect Chemother. 2003; 35:61–70.
32). Park SH, Choi SM, Chang YK, Lee DG, Cho SY, Lee HJ, et al. The efficacy of non-carbapenem antibiotics for the treatment of community-onset acute pyelonephritis due to extended-spectrum β -lactamase-producing Escherichia coli. J Antimicrob Chemother. 2014; 69:2848–56.
33). Chang R, Greene MT, Chenoweth CE, Kuhn L, Shuman E, Rogers MA, et al. Epidemiology of hospital-acquired urinary tract-related bloodstream infection at a university hospital. Infect Control Hosp Epidemiol. 2011; 32:1127–9.

34). Menashe G, Borer A, Yagupsky P, Peled N, Gilad J, Fraser D, et al. Clinical significance and impact on mortality of extended-spectrum beta-lactamase-producing Enterobacteriaceae isolates in nosocomial bacteremia. Scand J Infect Dis. 2001; 33:188–93.
35). Kim BN, Woo JH, Kim MN, Ryu J, Kim YS. Clinical implications of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae bacteraemia. J Hosp Infect. 2002; 52:99–106.
36). Wang F, Wu Y, Tang L, Zhu W, Chen F, Xu T, et al. Brain natriuretic peptide for prediction of mortality in patients with sepsis: a systematic review and meta-analysis. Crit Care. 2012; 16:R74.

37). Hur M, Kim H, Lee S, Cristofano F, Magrini L, Marino R, et al. Diagnostic and prognostic utilities of multimarkers approach using procalcitonin, B-type natriuretic peptide, and neutrophil gelatinase-associated lipocalin in critically ill patients with suspected sepsis. BMC Infect Dis. 2014; 14:224.

Fig. 1.
Schematic overview of the study. CPR: cardiopulmonary resuscitation; DNR: do-not-resuscitate.
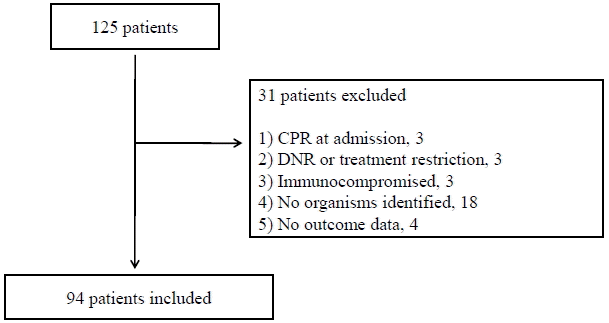
Fig. 2.
Rates of initial inappropriate antibiotic administration in patients with extended-spectrum β-lactamase, ESBL-negative multi-drug-resistant (ESBL-negative MDR), and non-ESBL/non-MDR organisms (42.1 % vs. 20.7% vs. 2.2%, respectively; ESBL vs. non-ESBL/non-MDR, p < 0.001; ESBL-negative MDR vs. non-ESBL/non-MDR, p = 0.012). ESBL: extended-spectrum β-lactamase; MDR: multidrug-resistant.
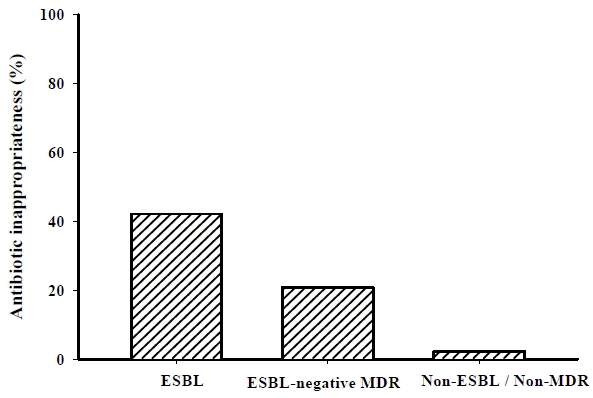
Fig. 3.
Kaplan-Meier curves to compare time intervals to appropriate antibiotic therapy among the three groups. Both patients with extended-spectrum β-lactamase (5.0 h [1.5–72.0 h]) and ESBL-negative multidrug-resistant (ESBL-negative MDR; 2.0 h [1.5–4.5 h]) organisms had a longer time to appropriate antibiotic treatment than those with non-ESBL/non-MDR organisms (2 h [1.5–3.0 h]; p < 0.001 and p = 0.032, respectively, by log-rank test). ESBL: extended-spectrum β-lactamase; MDR: multidrug-resistant.
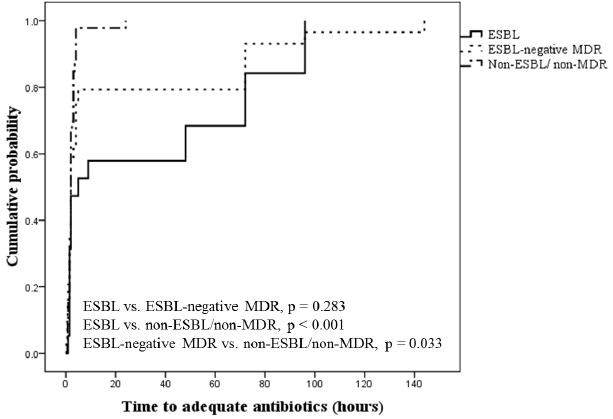
Fig. 4.
Hospital mortality rates in patients with extended-spectrum β-lactamase, ESBL-negative multidrug-resistant (ESBL-negative MDR), and non-ESBL/non-MDR organisms (31.6% vs. 10.3% vs. 10.9%, respectively; ESBL organisms vs. others, p = 0.033). ESBL: extended-spectrum β-lactamase; MDR: multidrug-resistant.
Table 1.
Patient characteristics
| Variables | N (%) |
|---|---|
| Age, yr | 73.0 (65.0–81.0) |
| Gender, M/F | 17/77 |
| Co-morbid illnesses | |
| Charlson comorbidity index | 5.5 (4.0–7.0) |
| Diabetes | 40 (42.6%) |
| Hypertension | 53 (56.4%) |
| COPD/BA | 3 (3.2%) |
| CVA | 29 (30.9%) |
| Heart disease | 14 (14.9%) |
| Liver cirrhosis | 5 (5.3%) |
| CKD | 16 (17.0%) |
| Cancer | 9 (9.6%) |
| SAPS II at admission | 47.0 (41.0–56.0) |
| Prior admission within one year | 52 (55.3%) |
| CA/HCA/HA | 55/25/14 |
| Bacteremia | 65 (69.1%) |
| Severe sepsis/Septic shock | 21 (22.3%)/57 (60.6%) |
| Catheter-associated UTI* | 20 (21.3%) |
* Urinary catheter for two weeks or longer. COPD: chronic obstructive pulmonary disease; BA: bronchial asthma; CVA: cerebrovascular accident; CKD: chronic kidney disease; SAPS: simplified acute physiology score; CA: community-acquired; HCA: healthcare-associated; HA: hospital-acquired; UTI: urinary traction infection.
Table 2.
Causal organisms of UTI sepsis
| Organisms | N (%) | Bacteremia | ESBL | MDR* |
|---|---|---|---|---|
| Escherichia coli | 66 (70.2%) | 46 (69.7%) | 14 (21.2%) | 21 (31.8%) |
| Proteus mirabilis | 7 (7.4%) | 7 (100.0%) | 3 (42.9%) | 1 (14.3%) |
| Klebsiella pneumoniae | 6 (6.4%) | 4 (66.7%) | 2 (33.3%) | 1 (16.7%) |
| Enterobacter aerogenes | 4 (4.3%) | 1 (25.0%) | 0 (0.0%) | 1 (25.0%) |
| Enterobacter cloacae | 1 (1.1%) | 1 (100.0%) | 0 (0.0%) | 1 (100.0%) |
| Enterococcus faecalis | 3 (3.2%) | 1 (33.3%) | 0 (0.0%) | 2 (66.7%) |
| Streptococcus agalatiae | 1 (1.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Viridans streptococcus | 1 (1.1%) | 1 (100.0%) | 0 (0.0%) | 0 (0.0%) |
| Citrobacter freundii | 1 (1.1%) | 1 (100.0%) | 0 (0.0%) | 0 (0.0%) |
| Acinetobacter baumannii | 1 (1.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Pseudomonas aeruginosa | 1 (1.1%) | 1 (100.0%) | 0 (0.0%) | 1 (100.0%) |
| Morganella morganii | 1 (1.1%) | 1 (100.0%) | 0 (0.0%) | 1 (100.0%) |
| Candida albicans | 1 (1.1%) | 1 (100.0%) | - | - |
Table 3.
Clinical and laboratory variables of survivors and non-survivors
| Variables | Survivors (n = 80) | Non-survivors (n = 14) | p value | p value* |
|---|---|---|---|---|
| Age, yr | 73.0 (65.3–80.8) | 73.5 (66.8–82.3) | 0.614 | - |
| Gender, M/F | 16/64 | 1/13 | 0.435 | - |
| Charlson comorbidity index | 5.0 (4.0–7.0) | 6.0 (4.8–8.0) | 0.241 | - |
| Laboratory findings† | ||||
| Hematocrit, % | 34.1 (29.1–37.9) | 28.1 (26.1–33.2) | 0.046 | 0.113 |
| Lactate, mmol/L | 3.8 (2.3–5.4) | 4.5 (3.3–10.5) | 0.079 | - |
| BNP, pg/ml | 236.2 (119.9–490.5) | 1102.6 (389.0–2234.4) | 0.001 | 0.024 |
| Troponin I, ng/ml | 0.03 (0.02–0.11) | 0.13 (0.04–0.22) | 0.074 | - |
| SAPS II at admission | 45.0 (40.0–51.8) | 60.5 (56.3–74.8) | < 0.001 | < 0.001 |
| Prior admission within one year | 41 (51.3%) | 11 (78.6%) | 0.058 | - |
| Catheter-associated UTI‡ | 17 (21.3%) | 3 (21.4%) | 0.988 | - |
| Hospital-acquired infection | 11 (18.3%) | 3 (21.4%) | 0.433 | - |
| Prior history of UTI within one year | 14 (17.5%) | 5 (35.7%) | 0.117 | - |
| Structural abnormalities or lesions | 25 (31.3%) | 5 (35.7%) | 0.762 | - |
| ESBL production | 13 (16.3%) | 6 (42.9%) | 0.033 | 0.046 |
| ESBL-negative MDR | 26 (32.5%) | 3 (21.4%) | 0.376 | - |
| Bacteremia | 55 (68.8%) | 10 (71.4%) | 1.000 | - |
| Inappropriate antibiotics | 10 (12.5%) | 5 (35.7%) | 0.044 | 0.210 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download