Abstract
The best management strategy for angiographically intermediated coronary artery diseases remains controversial. Lesions, when coupled with spasm, can lead to catastrophic results and cardiogenic shock. We report a case of a 62-year-old man who had an intermediate coronary artery disease presenting with cardiogenic shock due to coronary spasm during a preoperative period.
When an intermediate coronary artery disease (CAD) is revealed on coronary angiography (CAG), making a decision on how to treat as well as preparation for non-cardiac surgery is challenging. Prophylactic revascularization is supported only in the case of severe extensive ischemia.[1,2] Inducible ischemia caused by an intermediate CAD would be worsened when coupled with spasm. If we miss the possibility of variant angina pectoris in the patient who have intermediated CAD, using routine beta-blocker can be dangerous. We present a case of cardiogenic shock in a patient with an intermediate CAD in the preoperative exam, and who had been previously deferred for revascularization.
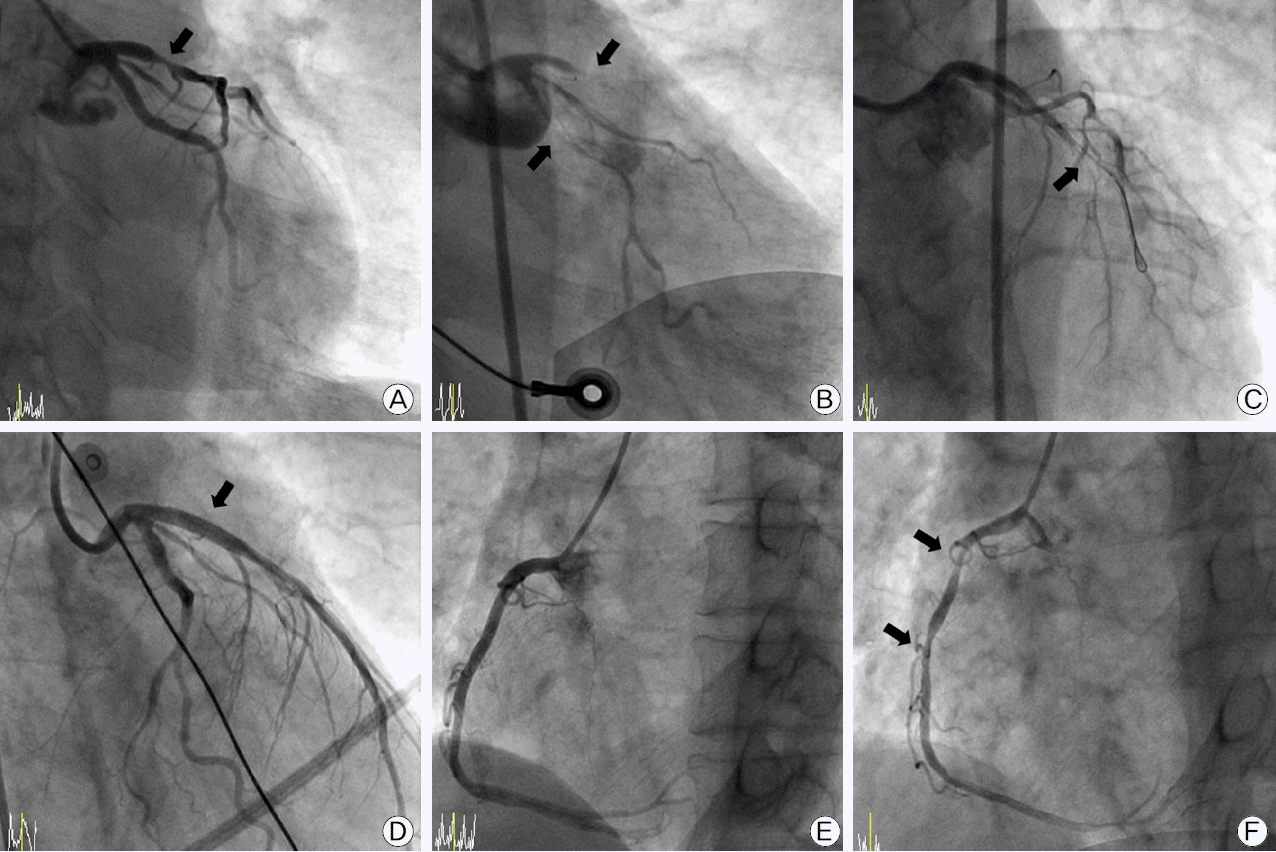
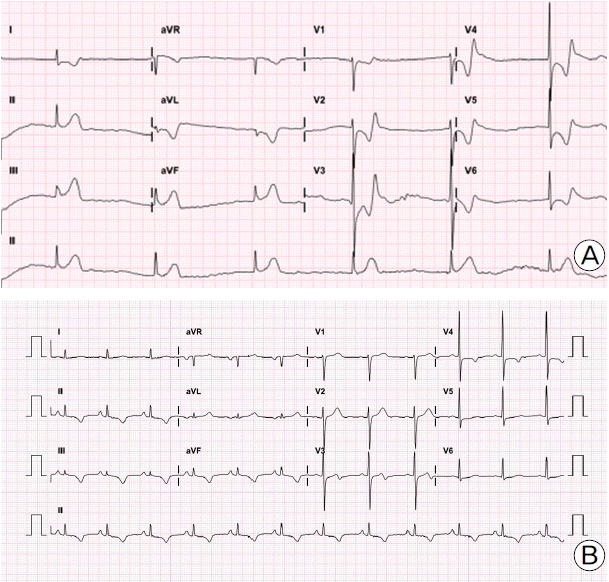
A 62 year-old Korean man was referred for perioperative risk assessment prior to gastrectomy for advanced gastric cancer. He was a smoker with a history of hypertension. His left ventricular systolic function was normal without abnormality in the regional wall motion. He had atypical, effort-unrelated substernal chest pain, and it was difficult to assess his functional capacity. Consequently, we prepared a coronary angiography to determine latent unstable angina pectoris, and the coronary angiography showed intermediate coronary artery disease (stenosis diameter 64% by Quantitative coronary angiography) (Fig. 1A). The patient was discharged without coronary intervention with a plan to undergo gastrectomy while being medicated with statin, low dose β-blocker (bisoprolol hemifumarate 2.5 mg qd), calcium channel blocker (amlodipine besylate 5 mg qd), and nitrate (isosorbide-5-mononitrate 20 mg bid). The following day, he was readmitted with worsening chest pain after drinking alcohol, and rapidly deteriorated into cardiogenic shock. The electrocardiogram (ECG) showed ST-segment elevation in leads II, III, and aVF, and ST-segment depression in leads I, aVL, and precordial leads, with junctional escape rhythm (Fig. 2A). Ventricular fibrillation occurred at the arrival to the catheterization laboratory, and it was reverted by defibrillation shock. The coronary angiography via right femoral artery revealed total and simultaneous occlusions at the proximal segment of both the left descending artery (LAD) and the left circumflex artery (LCX) (Fig. 1B). We assessed that multivessel spasm could even trigger cardiogenic shock and the total occlusion of the distal right coronary artery (RCA), and it was found that the LCX and a total occlusion of the LAD had arisen in this patient. An intra-aortic balloon pump was inserted via the left femoral artery, and intracoronary nitroglycerin of 200 μg was administered. After wiring into the LAD, balloon inflation (Ryujin® 2.5 × 10 mm, Terumo, Japan) was performed in the proximal LAD where the intermediate stenosis from the previous angiography was found. The subsequent angiography showed a diffuse spasm in downstream vessel (Fig. 1C). However, the patient was in a state of cardiogenic shock and it was intractable to inject intracoronary nitroglycerin and to inflate the balloon. Therefore, we decided to insert stent using a bare metal stent (Multi-Link Vision®, 3.5 × 18 mm; Abbott Vascular, USA) (Fig. 1D). Comparing to the previous angiography (Fig. 1E), the right coronary angiography following the intervention of the LAD revealed multi-segments spasm (Fig. 1F). To resolve a cardiogenic shock after stent insertion, we administered several times the intracoronary nitroglycerin injection, and then, ST-segment changes and rhythm were restored (Fig. 2B). The patient was treated with calcium channel blocker, nitrate, nicorandil, and statin, as well as aspirin and clopidogrel. The intra-aortic balloon pump was removed the next day. He was discharged. He under-went uneventfully 42 days after the coronary intervention withholding clopidogrel for 5 days before the planned gastrectomy surgery.
Prophylactic revascularization before a non-cardiac surgery is recommended only for those who have severe extensive ischemia or are in unstable cardiac conditions.[1,2] When the patient has an intermediate CAD and atypical symptoms, as in the present case, the decision of whether or not to treat can be very difficult. If a patient is not in an unstable cardiac condition, management with medications including statin and β-blocker is feasible. However, when the lesion is combined with spasm, it can become very serious and even worsened by concomitant use of β-blockers.
Coronary vasospasm is not an infrequent cause of ischemia in Asian population.[3] Particularly, the digestive track surgery could put patients at risk for spasm, refraining them from taking their oral medications.[4] One study reported 42 (0.05%) cases of cardiac event caused by vasospasm, among 77,745 patients undergoing non-cardiac surgery.[5] Although the frequency was very low during the peri-operative period, the results were fatal including 9 myocardial infarctions, 4 fatal arrhythmias, 29 unstable anginas, and 1 death. Interestingly, most of them had lower Revised Cardiac Risk Index (RCRI) score (0 or 1) classifying them as low risk.
Percutaneous coronary intervention (PCI) is not the preferred treatment for diffuse coronary artery spasm. Some studies recommend that when vasospastic angina is suspected, the non-invasive tests such as holter and hyperventilation test should be performed. If inappropriate response to drug therapy is observed, the invasive test such as ergonovine provocation test could be performed during coronary angiography. In addition, for patients with severe coronary artery disease, PCI and beta blocker are effective and actively considered. However, when the patient has an intermediate coronary artery disease in preoperative period, as in this case, ergonovine provocation test needs to be considered and it is better to refrain from the use of beta blocker.[6]
There are some studies reporting the use of stenting for the treatment of variant angina.[7,8] Interventional treatment in variant angina is still controversial due to complications that are difficult to avoid. There was a case in which the stent implantation provoked spasm.[9] Although implantable cardioverter defibrillators are often recommended in some of the patients with malignant arrhymias from intractable spasm, no agreement exists concerning the validity of this treatment.[6] In this case, the patient with already known intermediate coronary disease progressed into cardiogenic shock due to intractable vasospasm, so we decided to use stenting.
This case highlights the potential for diffuse, generalized vasospasm occurring in patients during preoperative period. Thus, physicians should consider coronary vasospasm in the differential diagnosis in the course of perioperative risk assessment. Coronary vasodilators, such as nitrates and/or calcium channel blockers, are preferable to β-blockers in such cases.
References
1). Fleisher LA, Beckman JA, Brown KA, Calkins Hugh, Chaikof EL, Feischmann KE, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol. 2009; 54:e13–e118.

2). Poldermans D, Bax JJ, Boersma E, Hert SD, Eeckhout E, Fowkes G, et al. Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J. 2009; 30:2769–812.

3). Pristipino C, Beltrame JF, Finocchiaro ML, Hattori R, Fujita M, Mongiardo R, et al. Major racial differences in coronary constrictor response between japanese and caucasians with recent myocardial infarction. Circulation. 2000; 101:1102–8.

4). Kurabayashi M, Okishige K, Asano M, Suzuki H, Shimura T, Iwai S, et al. Cardiopulmonary arrest caused by coronary spasm after coronary vasodilator withdrawal during the peri-operative period of gastrectomy. Intern Med. 2013; 52:81–4.

5). Nagayoshi Y, Kawano H, Kojima S, Soejima H, Kaikita K, Nakayama M, et al. Significance of coronary vasospasm in the perioperative management of non-cardiac surgery. Circ J. 2012; 76:1965–71.

6). JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2008). Circ J. 2010; 74:1745–62.
7). Jeong MH, Park JC, Rhew JY, Kang KT, Lee SH, Cho JH, et al. Successful management of intractable coronary spasm with a coronary stent. Jpn Circ J. 2000; 64:897–900.

8). Chu G, Zhang G, Zhang Z, Liu S, Wen Q, Sun B. Clinical outcome of coronary stenting in patients with variant angina refractory to medical treatment : a consecutive single-center analysis. Med Princ Pract. 2013; 22:583–7.
Fig. 1.
Coronary angiogram and percutaneous coronary intervention are shown. (A) Left coronary angiogram before cardiogenic shock is shown. (B) Left coronary angiogram after cardiogenic shock is shown. (C) Diffuse spasm in downstream vessel after wiring into the Left anterior descending coronary artery is shown. (D) Placing a bare metal stent is shown. (E, F) The right coronary angiogram is shown before and after cardiogenic shock. Arrows in (A-C) and (F) indicate lesions. Arrow in (D) points to stented segments.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download