Abstract
Background
Peripheral venous catheterization (PVC) is a less invasive and time consuming technique than central venous catheterization (CVC); however, for patients in circulatory collapse or receiving cardiopulmonary resuscitation (CPR), PVC cannot be achieved easily. CVC can provide not only a more effective administration route for medication, but also important hemodynamic information. Owing to the possibility of CPR interruptions and complications, CVC is recommended only after the failure of PVC. This observational study is aimed to evaluate the risks and benefits of CVC during CPR.
Methods
This retrospective observational study was performed in the emergency department (ED) of a university hospital. Adult patients without a pulse on arrival were consecutively enrolled if subclavian CVC was performed at the beginning of CPR. Patients who already had an established intravenous route or had severe chest injuries on arrival were excluded. Closed-circuit television was used to evaluate the frequency of compression interruption. The incidence of iatrogenic pneumothorax, an acute mechanical complication associated with subclavian CVC, was investigated using chest X-ray after CPR.
During CPR, central venous catheterization (CVC) is not recommended for drug delivery route, even if peripheral venous catheterization (PVC) cannot be achieved.[1] Because CVC may interrupt cardiopulmonary resuscitation (CPR), it does not have higher priority.[2] In addition, more than 15% of patients who undergo CVC experience complications such as pneumothorax.[3] For these reasons, CVC have not been considered as emergent vascular route. However, the PVC in emergent patients is not easy. The success rate of PVC was reported about 76%.[4] If CVC could be achieved in this case, the medication could be delivered, and the better quality of CPR could be possible rather than without any vascular route, and any medications.
Moreover, the central venous route has some unique advantages, though CVC can be associated with CPR interruption and complications. Compared with the peripheral venous route or the intraosseous route, higher peak drug concentrations are achieved with the central venous route, that is to say drug circulation time is shorter.[5,6] In addition, central venous oxygen saturation and coronary perfusion pressure can be monitored with a central line extending into the superior vena cava during CPR. These bio-chemical and hemodynamic information are useful for predicting return of spontaneous circulation, and for evaluating the success of the CPR.[7,8]
Unfortunately, it is not known yet how often the CVC interrupts chest compressions, and whether subclavian CVC increases the frequency of iatrogenic pneumothorax. These questions should be answered to compare the risk and benefit of performing CVC during CPR.
This study was a retrospective single-blind observational study, conducted in the emergency department (ED) of an urban teaching hospital with approximately 35,000 presentations a year. The study period was 6 months, from April to September 2011. During the period, patients who visited the ED without pulse and underwent CPR were consecutively enrolled. Patients, who already had intravascular catheter before arriving to the ED, were excluded from this study. The Patients with penetrating or blunt chest injuries might have induced hemothorax or pneumothorax, were excluded from evaluation for the frequency of iatrogenic pneumothorax.
All the cardiac arrest patients without obvious signs of death (e.g., decapitation, rigor mortis, or dependent lividity) were brought to the ED by paramedics in this local area. These paramedics were able to provide basic life support with advanced airway management and automated external defibrillation, but were not certified to administer any intravenous medications except hydration fluids. Usually, vascular access for delivery of CPR medication was provided for the arrest patients after ED arrival.
In our ED, not only PVC but also the subclavian CVC were performed independently and simultaneously for to prevent the delay or the failure to achieve vascular route during CPR. All physicians who performed CVC were emergency medicine residents over second grade, and had previously over than 20 times of CVC after book review and bedside teaching by faculty in the ED. All the nurses who performed PVC during CPR had an average of 4 years of work experience in ED. Other CPR procedures were performed according to established guidelines.[9]
The frequency of iatrogenic pneumothorax and hemothorax associated with subclavian CVC was the first primary outcome. A chest radiograph was obtained after CPR, and reviewed for the presence of pneumothorax and hemothorax. The frequency of CPR interruption, defined as greater than 10 seconds of compression interval time during procedures or interventions, was the second primary outcome.[10] Because the CPR registry and medical records did not contain the information needed to evaluate the chest compression pause, closed-circuit television (CCTV, WCS-0030, LevelOne, Taiwan) was utilized to evaluate the frequency of CPR interruptions.[11] The CCTV recording of the first 10 minutes in the ED was reviewed by two physicians who were not involved in the CPR. Each cause of interruption was also noted from the video, and reported as a secondary outcome.
All data were collected and managed with Microsoft Excel 2007 software (Microsoft Corporation, Santa Rosa, CA, USA). The median and interquartile ranges (IQR) of each outcome were statistically analyzed.
The Institutional Review Board of the hospital approved the study. Medical personnel, who were involved in the CPR, were not informed about the specific study purpose and design. The informed patient and medical personnel consent requirement was waived by the board (DKUH 2012-05-002). CCTV recording performed in the hospital was approved by the hospital quality assurance committee.
During the study period, 35 cardiac arrest patients presented to the ED and underwent CPR. The mean age of patients was 55.3 years (SD = 20.4 years), and 23 patients (65.7%) were male. (Fig. 1) Before ED arrival, four patients had undergone intravascular catheterization, and excluded from this study. The rest 31 patients underwent subclavian CVC in initial CPR. The five patients who had been related with severe chest injury were excluded from the evaluation of subclavian CVC related pneumothorax (Table 1).
Among 31 trials, subclavian CVC were successfully inserted in 28 patients, though in six patients more than one trial was necessary for successful catheterization (CVC success rate at first trial: 22/31, 71%). Despite several trails, central catheterization failed in 3 patients (CVC failure rate 9.7%, 3/31). The median time of catheterization were calculated from the patient arrival to the infusion and from the skin preparation. The results were 415 seconds (inter-quartile range [IQR]: 317.5–571.5 seconds), and 330 seconds (IQR: 271.25–403.75 seconds). In the same way, PVC succeeded at first trial in 17 patients (17/31, 54.8%), and did not achieved before the success of CVC in 9 patients (PVC failure rate 28.0%, 9/31). The median times of PVC procedure were 118 seconds (IQR: 8–148 seconds) and 38 seconds (IQR: 25.5–54.5 seconds) (Fig. 1).
Out of the 26 patients without severe chest injury, only one patient was related with iatrogenic pneumothorax after subclavian CVC. (1/26, 3.8%) CCTV videos of the CPRs were recorded, for 31 patients. These videos showed that there were 67 chest compression interruptions greater than 10 seconds. Among these interruptions there were, 13 pauses (median: 15 seconds, IQR: 13–20 seconds) in 10 patients that were associated with subclavian CVC (10/31, 32.3%). Other interruptions were caused by moving the patient from the ambulance to the CPR bed (28 pauses), checking the patient’s pulse with switching compressors (14 pauses), intubation (7 pauses), echocardiography (3 pauses), and other procedures (e.g., cricothyroidostomy, undressing). On average, there were 1.9 interruptions per patient (Fig. 1).
According to previous reports, the probabilities of pneumothorax associated with subclavian CVC range from 1.5% to 3.1% in critically ill patients, and the event with 3.0% probability has a 0.5470 (probability = 1 − 26C0 × 0.030 × 0.9726) chance of occurring once or more in 26 cases.[12] Thus, it might not be significantly different, though the comparison of the complication risk between ordinary condition and CPR is limited by the sample size.
In our study, the patient with iatrogenic pneumothorax visited ED for hypovolemic shock caused by hematemesis. The patient underwent more than five needle passes for CVC. According to one clinical report describing risk factors for complications and failures of subclavian CVC, the number of needle passes may be strongly associated with the rate of failure and complications. The complication rate increases from 4.3% with one pass to 24.0% with more than two passes.[13] Moreover, CPR itself can also cause pneumothorax about 8% of the time, and we could not distinguish from CVC induced pneumothorax in this study design.[14] It might show needle induced pneumothorax larger than real.
There was a report about CPR hands-off time in the ED. According to the report, initial assessment (45 cases), pulse check with switching compressors (45 cases), echocardiography (13 cases), defibrillation (9 cases), intubation (9 cases), X-ray (4 cases), CVC (1 case), needle thoracotomy (1 case), and backboard placement (1 cases) had interrupted the chest compression for more than 10 seconds in a total of 45 CPR subjects.[15] During pulse checking with switching compressors, echocardiography, and intubation, the interruptions of chest compression also observed in our study. Unlike the other interrupting causes which are performed in the same area with the chest compression, CVC itself did not have an influence on the chest compression. The CCTV video recording of the CPR revealed that, persons who were responsible for chest compressions had a tendency to stop and help the procedures. These interruptions could be reduced by educating and training of them not to stop compressing.
Although the supine chest x-ray has a sensitivity of only 35% to 75% for the detection pneumothorax, we inevitably reviewed chest radiographs to determine the frequency of pneumothorax.[16,17] Recently the chest ultrasound, which might be more useful than radiographs to identify pneumothorax, frequently utilized, however there were not precisely documented in the medical records.[18]
The success rate at first attempt of CVC was higher than that of PVC, and the median time of procedure is shorter in PVC than in CVC, however it is difficult to generalize this study results about the procedure success rate or the procedure time with the limited operators. The success rate and the median time may depend on the skill of operators who performed the procedure. In case of PVC, median procedure time, and the first trial success rate of other studies with leukemia patients, and emergent patients out of hospital were 1-2 minutes, and 26–28% respectively. In comparison with patients CPR results, which showed higher first trial success rate and longer procedural time. The operators who have placed > 50 central venous catheters or trained over two years were considered to have less than half complication rates.[19,20] The results could be improved with the training and preparing of procedure.
Even if interruptions or complications exist, making an effort to reduce interruptions and complications would be better than not using the advantages of CVC. After making these efforts, we are going to study with a large scale to show that CVC could reduce the failure rate of vascular access and improve without complications and CPR interruptions.
References
1). Deakin CD, Nolan JP, Soar J, Sunde K, Koster RW, Smith GB, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 4. Adult advanced life support. Resuscitation. 2010; 81:1305–52.

2). Neumar RW, Otto CW, Link MS, Kronick SL, Shuster M, Callaway CW, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010; 122(18 Suppl 3):S729–67.
3). Merrer J, De Jonghe B, Golliot F, Lefrant JY, Raffy B, Barre E, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001; 286:700–7.

4). Lapostolle F, Catineau J, Garrigue B, Monmarteau V, Houssaye T, Vecci I, et al. Prospective evaluation of peripheral venous access difficulty in emergency care. Intensive Care Med. 2007; 33:1452–7.

5). Kuhn GJ, White BC, Swetnam RE, Mumey JF, Rydesky MF, Tintinalli JE, et al. Peripheral vs central circulation times during CPR: a pilot study. Ann Emerg Med. 1981; 10:417–9.

6). Emerman CL, Pinchak AC, Hancock D, Hagen JF. Effect of injection site on circulation times during cardiac arrest. Crit Care Med. 1988; 16:1138–41.

7). Paradis NA, Martin GB, Rivers EP, Goetting MG, Appleton TJ, Feingold M, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990; 263:1106–13.

8). Rivers EP, Martin GB, Smithline H, Rady MY, Schultz CH, Goetting MG, et al. The clinical implications of continuous central venous oxygen saturation during human CPR. Ann Emerg Med. 1992; 21:1094–101.

9). Neumar RW, Otto CW, Link MS, Kronick SL, Shuster M, Callaway CW, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010; 122(18 Suppl 3):S729–67.
10). Kramer-Johansen J, Edelson DP, Abella BS, Becker LB, Wik L, Steen PA. Pauses in chest compression and inappropriate shocks: a comparison of manual and semi-automatic defibrillation attempts. Resuscitation. 2007; 73:212–20.

11). Park HS, Jung SK, Kim HC. Accuracy of the cardiopulmonary resuscitation registry in an emergency department. Emerg Med J. 2012; 29:287–9.

12). McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003; 348:1123–33.

13). Mansfield PF, Hohn DC, Fornage BD, Gregurich MA, Ota DM. Complications and failures of subclavian-vein catheterization. N Engl J Med. 1994; 331:1735–8.

14). Kim EY, Yang HJ, Sung YM, Cho SH, Kim JH, Kim HS, et al. Multidetector CT findings of skeletal chest injuries secondary to cardiopulmonary resuscitation. Resuscitation. 2011; 82:1285–8.

15). Park JB, Jo YI, Cho YS, Choi HJ, Kang BS, Lim TH, et al. Factors affecting cardiopulmonary resuscitation hands-off time in an emergency room. J Korean Soc Emerg Med. 2012; 23:221–8.
16). Lichtenstein DA, Mezière G, Lascols N, Biderman P, Courret JP, Gepner A, et al. Ultrasound diagnosis of occult pneumothorax. Crit Care Med. 2005; 33:1231–8.

17). Blaivas M, Lyon M, Duggal S. A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic pneumothorax. Acad Emerg Med. 2005; 12:844–9.

18). Johnson A. Emergency department diagnosis of pneumothorax using goal-directed ultrasound. Acad Emerg Med. 2009; 16:1379–80.

19). Sznajder JI, Zveibil FR, Bitterman H, Weiner P, Bursztein S. Central vein catheterization. Failure and complication rates by three percutaneous approaches. Arch Intern Med. 1986; 146:259–61.

20). Fares LG 2nd, Block PH, Feldman SD. Improved house staff results with subclavian cannulation. Am Surg. 1986; 52:108–11.
Fig. 1.
Results of subclavian CVC and PVC for OHCA patient at emergency department. OHCA: out of hospital cardiac arrest; IV: intravascular; SD: standard deviation; VC: venous catheterization; CVC: central venous catheterization; PVC: peripheral venous catheterization; IQR: inter- quartile range.
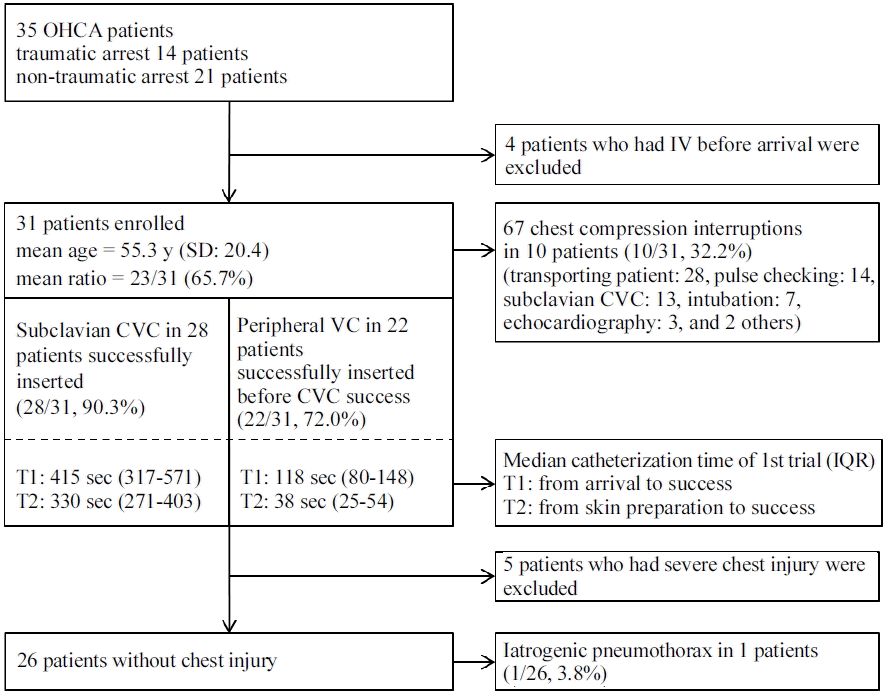
Table 1.
Injury mechanisms of traumatic arrest patients




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download