Abstract
It is well known that external chest compression during cardiopulmonary resuscitation is frequently associated with various complications. These complications predominantly involve trauma to the heart, lungs, and chest wall, whereas cases involving intra-abdominal injury are much less frequent. The present report describes a rare case of a female patient with severe hemoperitoneum associated with liver injury after cardiopulmonary resuscitation. Although emergent angiography and embolization of the hepatic artery were performed and transfusion of various kinds of blood products was done continuously, the patient expired the next day.
It is well known that external chest compression during external cardiac compression is frequently associated with various complications.[1–3] There are many reports referring predominantly to CPR-related trauma to the heart, lungs, and chest wall such as the rib bone and sternum, as these are frequent. In comparison, there are few data regarding the intra-abdominal injury due to external cardiac compression, as this is a rare event. Intra-abdominal resuscitation-associated complications, including hepatic, splenic or various gastrointestinal injuries, are found in some autopsy reports.[4–6] The present report describes a rare case involving a patient with massive hemoperitoneum by the liver injury after cardiopulmonary resuscitation.
A 72-year-old female was admitted with post-resuscitation status after sudden arrest. The patient had experienced several recent episodes of chest pain that lasted for a few minutes. She had been diagnosed with vasospastic angina 4 years earlier and treated with medication. However, she ran short of medications and has not taken medications for 1 week. Because the patient visited the hospital and collapsed within hospital, cardiopulmonary resuscitation (CPR) was performed immediately by doctors for 10 minutes, leading to return of spontaneous circulation (ROSC). After ROSC, blood pressure (BP) and heart rate were 79/50 mmHg and 134/min, respectively. Mechanical ventilation and vasopressor (norepinephrine) were applied.
Transthoracic echocardiography revealed the preserved left ventricular systolic function without regional wall motion abnormality. Emergent coronary angiography revealed no significant lesion in all three major coronary arteries. Initial laboratory test findings were as following: white blood cell count 15.0 × 109/L (neutrophil 87%), hemoglobin 15.1 g/dl, platelet count 163 × 109/L, AST 121 IU/L, ALT 187 IU/L, pH 7.13, PaCO2 37.6 mmHg, PaO2 140 mmHg, HCO3− 12.6 mmol/L, SaO2 99%. Blood urea nitrogen (BUN), serum creatinine, albumin, partial thromboplastin time (PTT), and prothrombin time (PT) were all within normal limits.
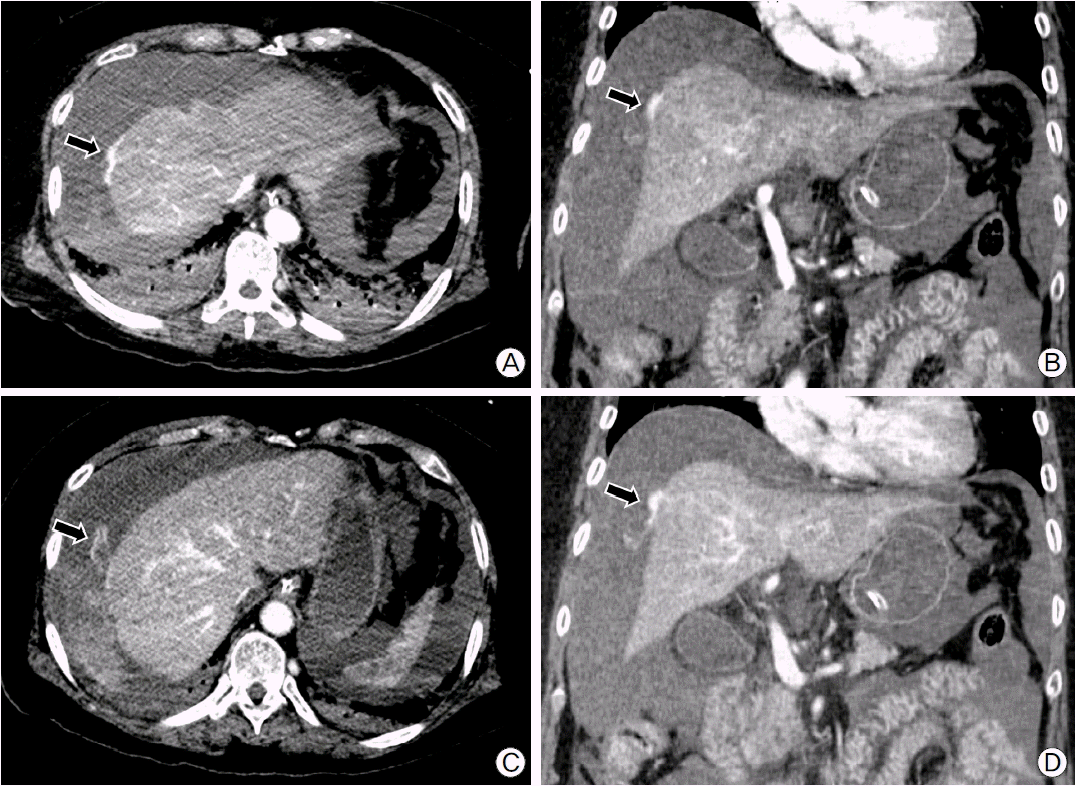
After admission to intensive care units, the patient’s abdomen was getting rapidly distended and BP was decreased to 61/38 mmHg. Follow-up laboratory test findings showed rapid drop in hemoglobin level (9.8 g/dl) and coagulation abnormalities (PTT 200 sec [26.8–40.6], PT 17.2 sec [9.2–12.3], fibrinogen 97 mg/dl [200–400], thrombin time 20.4 sec [13.0–18.0], antithrombin III 44% [80–120], and fibrin degradation products 44.1 ug/ml [0.0–5.0]). Fluid get from diagnostic paracentesis was bloody and hematocrit was 32%. An abdominal computed tomography demonstrated large amount of hemoperitoneum with focal laceration and contrast extravasation at the surface of liver, suggesting subcapsular active bleeding (Fig. 1).
Emergent angiography and blind embolization of the right and middle hepatic artery were performed empirically, since no definite bleeding focus was detected. Massive transfusion of various kinds of blood products, including packed red blood cells, platelet concentrates, and fresh frozen plasma, was done continuously, but the patient was getting hemodynamically unstable. As hypoxia, oliguria, and metabolic acidosis got worse, continuous veno-venous hemodiafiltration and veno-venous extracorporeal membrane oxygenation were applied. Despite all these efforts, the patient expired the next day.
This case report describes a female patient with severe hemoperitoneum associated with the liver injury after CPR. Although CPR was performed by skilled doctors for relatively short time, massive bleeding was not controlled and she expired finally.
It has been suggested that intra-abdominal complications, including liver injury, occur mostly because of an inappropriate technique of chest compression during CPR.[1,7] Patients resuscitated from cardiac arrest may have suffered minor liver injury and then deteriorate after systemic anticoagulation or thrombolytic treatment. Therefore, patients treated with thrombolytics or anticoagulants may be vulnerable to a visceral hemorrhage when these patients need CPR. On the other hand, spontaneous liver hematoma and rupture without prior resuscitation following thrombolytic treatment also have been described.[8] If either chest compressions or compromised hemostasis alone may cause liver bleeding, it seems obvious that the risk is even more increased when both conditions coexist. As the clinical presentation of liver injury after CPR often lacks obvious symptoms and signs, diagnosis is difficult, although it will influence both prognosis and further medical management of the patient.[1]
A prior study reported that major liver injury (rupture/laceration, hemorrhage/hematoma) was found just in 15 of 2558 cardiac arrest victims (0.6%) for 14 years. The site of injury was mostly the left liver lobe in 11 of 15 (73%).[9] Although hemostasis was compromised in 13 patients, no patient died from bleeding due to liver injury. This study confirms that major liver injury is an infrequent complication of CPR, and if managed appropriately, scarcely appears to influence overall outcome. In our case, however, the site of injury was infrequently the right liver lobe and the patient died from bleeding due to liver injury in spite of emergent angiography and embolization of hepatic artery. Although surgical intervention is the treatment of choice for stopping profusely bleeding liver injury,[9] our patient did not underwent surgery due to hemodynamic instability and severe coagulopathy. Instead, emergent embolization of hepatic artery was performed, but it was not effective in stopping a profuse bleeding.
The liver injury most often occurs in the left lobe.[9] An important reason of injury to the liver may be the close anatomical relation between the liver and the incorrect placement of hands for performing chest compressions, namely over the xiphoid bone.[10,11] It was also suggested that the liver injury was associated not only with choosing an improper point for hand placement, but also with exertion of excessive pressure during chest massage, leading to severe crush injury of viscera.[12] However, these complications have also been described, even when light chest compressions were performed at a correct point.[4,13] It may mean that these complications cannot always be avoided.
Hemodynamic instability is considered a leading symptom of intra-abdominal bleeding.[9] However, this is a common presentation in patients in the post-resuscitation phase. A quantifiable measurement like the hematocrit might be more useful. Hematocrit is easily monitored by regularly checking the blood count, and in case of significant change should lead to further diagnostic workup.
The benefit of immediate and continuous chest compressions by far outweighs the infrequent and treatable complication of major liver injury. Awareness should be especially high when chest compression is performed in patients treated with thrombolytics, antithrombotic or antiplatelet agents, irrespective whether CPR is performed in- or out-of-hospital, by professionals or by lay by-standers. Clinical assessment, low and dropping hematocrit should trigger suspicion; bedside abdominal sonography to detect free intra-abdominal fluid or signs of direct liver injury should be the next diagnostic step. Repeat examination or imaging by CT/MR may be necessary. In conclusion, we would like to emphasize timely diagnosis and appropriate management of CPR associated major liver injury.
REFERENCES
1). Schneider A, Bottiger BW, Popp E. Cerebral resuscitation after cardiocirculatory arrest. Anesth Analg. 2009; 108:971–9.

2). Busto R, Dietrich WD, Globus MY, Ginsberg MD. Postischemic moderate hypothermia inhibits ca1 hippocampal ischemic neuronal injury. Neurosci Lett. 1989; 101:299–304.

3). Busto R, Dietrich WD, Globus MY, Valdés I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab. 1987; 7:729–38.

4). Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: A prospective, randomized study. Crit Care Med. 1993; 21:1348–58.
5). Rosen J, Tuchek JM, Hartmann JR. Liver laceration in the hemodynamically unstable post-cardiac massage patient: Early recognition and management-case report. J Trauma. 1999; 47:408–9.
6). Ziegenfuss MD, Mullany DV. Traumatic liver injury complicating cardio-pulmonary resuscitation. The value of a major intensive care facility: a report of two cases. Crit Care Resusc. 2004; 6:102–4.
7). Coimbra C, Wieloch T. Moderate hypothermia mitigates neuronal damage in the rat brain when initiated several hours following transient cerebral ischemia. Acta Neuropathol. 1994; 87:325–31.

8). Krammer B, Steiner M, Burstein C, Voss W, Kroger JC, Zillig D, et al. [Spontaneous, massive liver hemorrhage as a complication of thrombolysis with ultra-high dose streptokinase in deep thrombophlebitis]. Vasa. 1994; 23:373–6.
9). Meron G, Kurkciyan I, Sterz F, Susani M, Domanovits H, Tobler K, et al. Cardiopulmonary resuscitation-associated major liver injury. Resuscitation. 2007; 75:445–53.

10). Zbar RI. Liver laceration after cardiopulmonary resuscitation: a case report. Heart Lung. 1993; 22:463.
11). Morgan RR. Laceration of the liver from closed-chest cardiac massage. N Engl J Med. 1961; 265:82–3.

12). Umach P, Unterdorfer H. Massive organ injuries resulting from resuscitation measures. Beitr Gerichtl Med. 1980; 38:29–32.
13). Lignitz E, Gillner E, May D. [Complications of resuscitative measures with special regard to liver damage (author's transl)]. Prakt Anaesth. 1977; 12:523–6.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download