Abstract
Tachycardia-induced cardiomyopathy is caused by persistent tarchyarrhythmias and is characterized by ventricular systolic dysfunction and congestive heart failure. Tachycardia-induced cardiomyopathy is usually reversible via treatment. The cornerstone in the management of disease in these patients is to achieve a normal heart rate. We report a torsades de pointes during treatment of tachycardia-induced cardiomyopathy. Intravenous magnesium sulfate and potassium were administrated, but torsades de pointes was repeated. After overdrive right ventricular pacing, torsades de pointes was terminated. Careful monitoring of the QT interval and serum electrolyte and drug levels in such patients is warranted during treatment of tachycardia-induced cardiomyopathy.
Tachycardia-induced cardiomyopathy (TC) is caused by sustained arrhythmia and typically characterized by left ventricular systolic dysfunction and congestive heart failure. Early treatment for TC includes the use of various drugs, including diuretics, anticoagulant, β-blocker, in combination with antiarrhythmic drugs. However, the combination medical therapy is known to increase the risk of prolonged QT interval, electrolyte imbalance and heart failure, which lead to serious arrhythmia such as torsades de pointes (TdP) unless they are treated properly. We present a case of TdP occurred in a patient being treated for TC and review other cases from the literature.
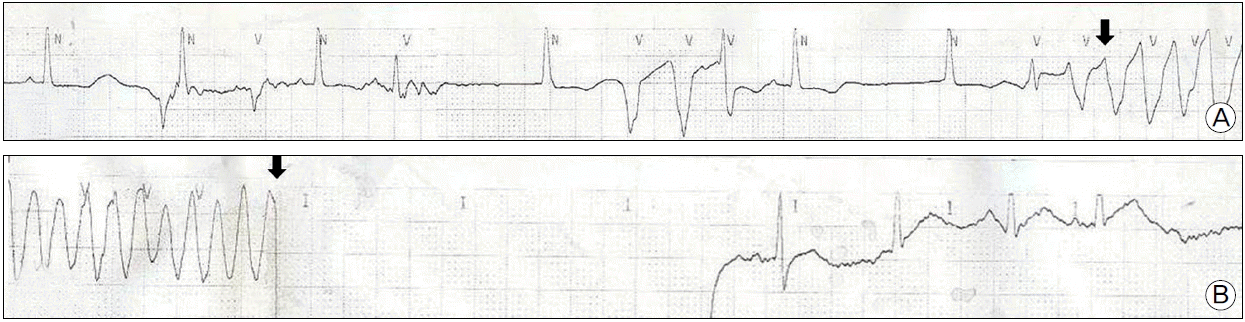
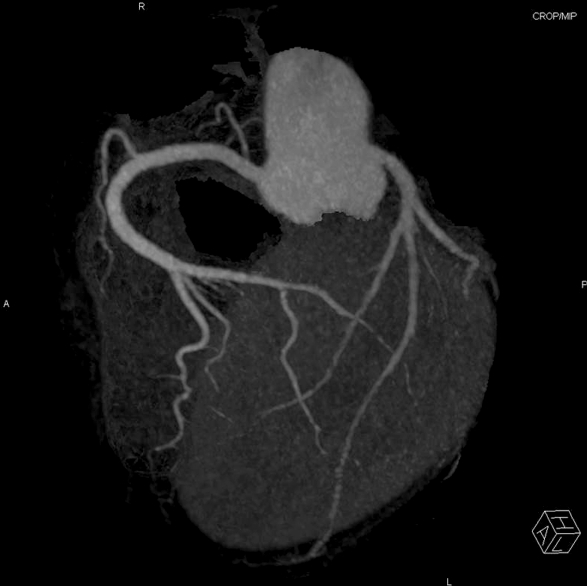
A 45-year-old woman presented with palpitations occurring three to four times per month over a period of one year and difficulty in breathing that lasted for one month. The patient did not undergo a cardiac evaluation before but had a history of hysterectomy due to uterine myoma. On admission, the patient had a blood pressure of 119/104 mmHg, pulse rate of 169 beat per minute (bpm), respiration rate of 22 per minute and temperature of 37°C. Complete blood cell count revealed leukocyte of 11,300/mm3 (neutrophils 8,630/mm3) and hemoglobin of 14.9 g/dl. Serum sodium and potassium were 142 mmol/L, and 4.2 mmol/L, respectively. And brain natriuretic peptide was elevated to 1120 pg/ml (normal range: 164 pg/ml or lower). Thyroid function test were normal showing T3 54 ng/ml, free T4 1.33 ng/ml and TSH 0.92 μIU/ml. However, chest radiography showed cardiomegaly and bilateral pulmonary edema (Fig. 1a). Also, electrocardiography showed atrial fibrillation with a heart rate of 172 beats/min, and the adjusted QT interval was 450 msec (Fig. 2a). Echocardiogram detected severe left ventricular (LV) systolic dysfunction with LV ejection fraction of 15% and mild left ventricular dilation indicated by LV end-diastolic dimension of 59 mm (Fig. 3a). The patient was diagnosed with tachycardia-induced cardiomyopathy and given digoxin, furosemide, spironolactone, angiotensin converting enzyme inhibitor in combination with warfarin and heparin after being admitted to an ICU. On the first day after admission, her digoxin level was 0.51 ng/ml (normal range 0.8–2.4 ng/ml). As atrial fibrillation was still present on the second day after admission, a transesophageal echocardiography was performed. After we checked no thrombus in left atrium and left atrial appendage, amiodarone (60 mg/hr) was infused intravenously to restore sinus rhythm. To achieve cardioversion of atrial fibrillation sustained until the third day after admission, electric shock was delivered at initial energy of 100 J, followed by second energy of 150 J. Because electrical cardioversion was not successful, the patient was treated with oral amiodarone. On 4th day of admission, atrial fibrillation was converted to artial flutter with 3:1 conduction (heart rate of 72), and the adjusted QT interval increased to 548 msec (Fig. 2b). On the afternoon of the same day, the patient developed TdP and fell into a stuporous mentality, and her blood pressure was not checkable, calling for an immediate defibrillation (Fig. 4b arrow). As a result, arrhythmia was converted to normal sinus rhythm and vital signs became stable (Fig. 4). Low serum potassium level (3.0 mmol/L) was corrected by intravenous potassium and magnesium was also infused. Additionally, amiodarone and digoxin were withdrawn for the possible cause of TdP. However, artial flutter lasted continuously with atrioventricular block, and it resulted in the recurrence of TdP. Temporary over-drive pacing was therefore provided at the rate of 100 bpm by implanting a pacemaker into the right ventricle. No further TdP occurred and the patient was given medications in which amiodarone was excluded but carvedilol newly added. A chest radiography showed improvement in the pulmonary edema (Fig. 1b). Electrocardiography revealed normal sinus rhythm with premature atrial contraction. Also, coronary angioCT showed normal coronary artery (Fig. 5). Brain natriuretic peptide level dropped to 230 pg/ml (normal range :164 pg/ml or below) on 15th day of hospitalization and LV ejection fraction improved to 18.3% based on initial echocardiogram (Fig. 3b). The patient is undergoing outpatient follow-up after being discharged.
Tachycardia-induced cardiomyopathy (TC) can result in LV systolic dysfunction and dilatation and eventually heart failure because of sustained supraventricular tachycardia or ventricular tachycardia. TC is reversible when the heart beat is conversed to normal rate. The prevalence of arrhythmia for TC has not been established, patients with atrial fibrillation are most frequently reported.[1,2] In the absence of standard diagnostic criteria of TC, the physician needs to suspect TC in the presence of LV systolic dysfunction and sustained tachyarrhythmia. The ultimate goal of therapies for TC is to restore normal sinus rhythm.[3] Conversion to normal heart beat leads to symptomatic improvement by increasing LV ejection fraction and decreasing LV end-diastolic and LV end-systolic volumes. Arrhythmia treatment options can vary with individual conditions but include antiarrhythmic drugs, electrical conversion, pacemaker, and implantable cardioverter defibrillator. All these options are intended to restore normal heart rhythm as it improves ventricular function.[1,4,5] However, ventricular functional recovery is varying among patients after tachyarrhythmia is terminated or managed.[6]
In this case, the patient had hemodynamically stable atrial fibrillation of more than 48-hours duration and systolic heart failure presumed to be caused by tachycardia-induced cardio- myopathy. As we checked no thrombus in left atrium and left atrial appendage using transesophageal echocardiography, pharmacological conversion to sinus rhythm was performed by using amiodarone in addition to heparin. However, the patient’s atrial fibrillation was persistent for 24 hours despite drug therapy for restoration of sinus rhythm. Electrical cardioversion also failed to terminate atrial fibrillation. Then pharmacological intervention was instituted again for two days. In short, the patient was taking digoxin for the treatment of heart failure and given amiodarone additionally for conversion to sinus rhythm.
According to the 2013 American Heart Association guidelines for atrial fibrillation treatment, the combination of digoxin and amiodarone or sotalol for conversion to sinus rhythm can have harmful effects.[7] However such a warning can be oversighted in the presence of severe systolic heart failure caused by TC as described in this case.
Amiodarone is an effective class III antiarrhythmic drug that prolongs repolarization period and increases QT interval up to 20% from baseline QT interval. Like Class Ia antiarrhythmic drugs, amiodarone can prolong QT interval but it can also inhibit depolarization by acting on calcium channels. As a result, it is known to be safe compared to other antiarrhythmic drugs and its incidence rate of TdP is estimated at less than 1%.[8] However, the use of amiodarone in combination with digoxin increases the risk of arrhythmia. Especially, the onset of TdP is much more likely to occur within four days when amiodarone is administered.[9]
Risk factors for TdP include women gender, myocardial infarction, heart failure, cardiomyopathy, valvular heart disease, the use of antiarrhythmic drugs, bradyarrhythmias, low serum potassium, low serum magnesium, and family history of QT interval prolongation.[10] Intravenous magnesium is considered standard therapy for the onset of TdP regardless of serum concentration. Serum potassium concentration should be maintained at upper limit of the normal range (4.5–5 mmol/L). Hemodynamically unstable patients may require an immediate defibrillation. If patients does not respond to pharmacological intervention, ventricular overdrive pacing can be used as effective treatment option.[11]
In this case, we suggest the co-administration of digoxin and amiodarone as the primary cause of TdP and low serum potassium, heart failure, women gender and the use of diuretics also could be risk factors. Combination medications are widely used for early treatment of TC caused by atrial fibrillation. However, these drugs have potential risks of developing TdP. Careful monitoring of ECG changes, drug level of digoxin and electrolyte level is therefore recommended for preventing malignant arrhythmia such as TdP.
REFERENCES
1). Lishmanov A, Chockalingalingam P, Senthilkumar A, Chockalingam A. Tachycardia-induced cardiomyopathy: evaluation and therapeutic options. Congsestive Heart Fail. 2010; 16:122–6.

2). Redfield MM, Kay GN, Jenkins LS, Mianulli M, Jensen DN, Ellenbogen KA. Tachycardia-related cardiomyopathy: a common cause of ventricular dysfunction in patients with atrial fibrillation referred for atrioventricular ablation. Mayo Clin Proc. 2000; 75:790–5.

4). Steinhoff JP, Sheahan RG. Tachycardia-induced cardiomyopathy: atrial fibrillation and congestive heart failure. Am J Med Sci. 2005; 329:25–8.
5). Jeong YH, Choi KJ, Song JM, Hwang ES, Park KM, Nam GB, et al. Diagnostic approach and treatment strategy in tachycardia-induced cardiomyopathy. Clin Cardiol. 2008; 31:172–8.

6). Mohamed HA. Tachycardia-induced cardiomyopathy (Tachycardiomyopathy). Libyan J Med. 2007; 2:26–9.

7). Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, et al. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013; 61:1935–44.
8). Hohnloser SH, Klingenheben T, Singh BN. Amiodarone-associated proarrhythmic effects. A review with special reference to torsade de pointes tachycardia. Ann Intern Med. 1994; 121:529–35.

9). Bajaj BP, Baig MW, Perrins EJ. Amiodarone-induced torsades de pointes: the possible facilitatory role of digoxin. Int J Cardiol. 1991; 33:335–7.

10). Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003; 89:1363–72.

11). Gupta A, Lawrence AT, Krishnan K, Kavinsky CJ, Trohman RG. Current concepts in the mechanisms and management of drug-induced QT prolongation and torsades de pointes. Am Heart J. 2007; 153:891–9.
Fig. 1.
Chest radiography showed (A) cardiomegaly with bilateral pulmonary edema at 1st day of admission and (B) decreased heart size as compared to that of 1 day of admission and disappearance of pulmonary edema at 15th day of admission.
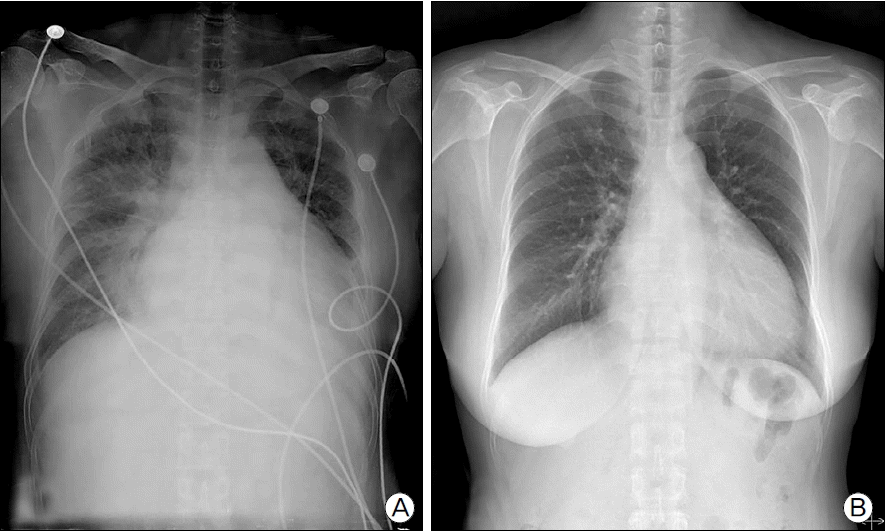
Fig. 2.
12-lead electrocardiogram (ECG) showed (A) atrial fibrillation with rapid ventricular response at admission and (B) atrial flutter with 3:1 atrioventricular conduction with markedly prolonged corrected QT interval at 4th day of admission.
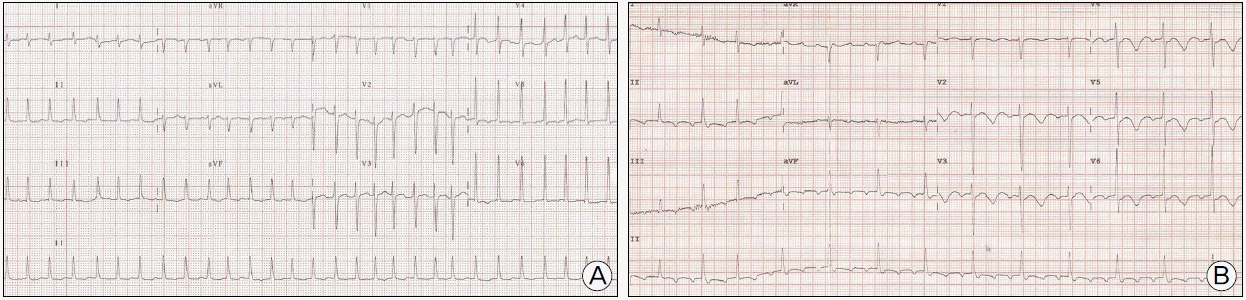
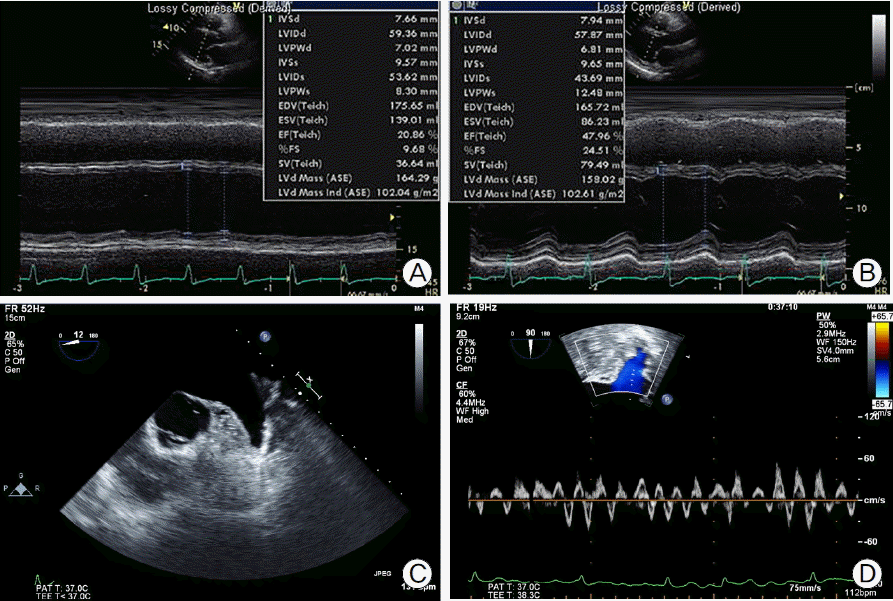
Fig. 3.
Transthoracic echocardiogram showed (A) severe LV systolic dysfunction with wall thinning at admission and (B) mildly improved LV systolic dysfunction at 15th day of admission. Transesophageal echocardiogram showed (C) no evidence of thrombus in LA appendage and (D) decreased LA appendage inflow and outflow pattern. LA: left atrium, LV: left ventricle.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download