Introduction
Hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome is a genetic syndrome resulting from germline mutations in fumarate hydratase (FH) [
1]. This autosomal dominant condition is characterized by cutaneous leiomyomas, early-onset multiple uterine leiomyomas, and an aggressive form of type 2 papillary renal cell carcinoma (RCC) [
2,
3]. Although RCC arising in HLRCC syndrome has been described as type 2 papillary RCC, the recently updated World Health Organization (WHO) 2016 genitourinary cancer classification added RCC in HLRCC as a new entity, HLRCC-associated RCC [
4].
More than 180 families with FH germline mutations have been reported [
3] but the exact incidence of HLRCC syndrome or HLRCC-associated RCC remains unknown as this entity has had a low awareness among urologists, medical oncologists, and pathologists until today. A considerable proportion of patients with HLRCC-associated RCC might have been diagnosed and treated as having sporadic papillary RCC and even after correct diagnosis, there has been no specific treatment option for advanced HLRCC-associated RCC. The most commonly used treatment might be mammalian target of rapamycin (mTOR) inhibitors and vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR TKIs), similar to the treatment of non-clear cell RCC [
5].
With the accumulation of pathobiological knowledge underlying HLRCC-associated RCC, there have been attempts at mechanism-based treatment for HLRCC-associated RCC. FH-deficient kidney cancer is characterized by impaired Krebs cycle and oxidative phosphorylation, thus depends on glucose for adenosine triphosphate generation by aerobic glycolysis (Warburg effect). Increased oxidative stress and/or increased levels of fumarate inhibit hypoxia-inducible factor (HIF) prolyl hydroxylase which facilitates ubiquitinmediated degradation of HIF, resulting in HIF stabilization. Accumulation of HIF leads to increased transcription of vascular endothelial growth factor (VEGF) [
2]. Bevacizumab can inhibit VEGF-mediated tumor vasculature. In a meanwhile, epidermal growth factor receptor (EGFR) signaling promotes aerobic glycolysis through the phosphoinositide 3-kinase/AKT or RAS/ mitogen-activated protein kinase pathway [
6], and EGFR TKI erlotinib reverted aerobic glycolysis in cancer cell line [
7].
Against this backdrop, phase II clinical trials evaluating the efficacy and safety of the combination of bevacizumab plus erlotinib (AVATAR trial, NCT01130519) and the combination of vandetanib plus metformin (NCT02495103) are underway. An interim analysis of the AVATAR trial of bevacizumab plus erlotinib showed an impressive objective response rate (ORR) of 65% in patients with HLRCC-associated RCC [
8] and a median progression-free survival (PFS) of 24.2 months. This promising result led to the recommendation of bevacizumab plus erlotinib for the treatment of HLRCC-associated RCC in the 2018 National Comprehensive Cancer Network (NCCN) guidelines [
9].
In Korea, however, HLRCC-associated RCC has only recently been recognized by physicians and, to our knowledge, there are currently no formal reports on Korean patients with this disease. Therefore, the outcome of bevacizumab plus erlotinib therapy in Korean patients is not known. For this reason, we retrospectively collected data on patients with HLRCC in Korea and to evaluate the efficacy and safety of the bevacizumab plus erlotinib combination treatment.
Materials and Methods
1. Patient selection
We retrospectively reviewed consecutive patients diagnosed with HLRCC-associated RCC and treated between July 2014 and July 2018 in three academic hospitals (Asan Medical Center, Gil Medical Center, and Seoul National University Hospital) in South Korea. The selection criteria for analysis were as follows: diagnosis of HLRCC-associated RCC, advanced stage (recurrent, locoregionally advanced unresectable, or metastatic disease), treatment with bevacizumab (10 mg/kg every 2 weeks intravenously) plus erlotinib (150 mg p.o daily) for HLRCC-associated RCC irrespective of line of treatment, and adequate clinical information and baseline and follow-up imaging.
Definitive diagnosis of HLRCC-associated RCC was confirmed by FH germline mutation test by polymerase chain reaction (PCR)–based sequencing methods from peripheral blood leukocytes. The criteria for proceeding to germline FH tests were as follows: suggestion of HLRCC-associated RCC in pathologic examination, diagnosis of papillary-type RCC under 40 years of age, pathologically confirmed cutaneous leiomyomatosis, multiple early-onset symptomatic uterine myoma in female patients or in family members of male patients, or family history of HLRCC syndrome.
2. Data collection and analysis
We collected the clinicopathological data of these patients from electronic medical record systems. Data on their baseline demographic characteristics, radiologic response and time-to-progression (TTP) to previous systemic treatments and bevacizumab plus erlotinib, adverse events related to bevacizumab plus erlotinib, and survival data were also gathered. Response evaluation according to Response Evaluation Criteria in Solid Tumors ver. 1.1 was performed by a comprehensive review of baseline and follow-up imaging. Usual radiographic evaluations were assessed every 2-3 months by computed tomography of the chest, abdomen, and pelvis but other image techniques were used at the discretion of the attending physicians. Safety was evaluated according to the Common Terminology Criteria for Adverse Events ver. 4.0, from the National Cancer Institute.
3. Statistical analysis
Patient characteristics and toxicity were summarized descriptively. The primary endpoint was ORR and the secondary endpoints were PFS, OS, and safety. Kaplan-Meier analysis was used to determine the time-to-event endpoints. PFS was defined as the time from treatment initiation to the date of documentation of disease progression or death from any cause, whichever came first, while OS was calculated as the time from treatment initiation to death from any cause. Cases in which progression and/or death did not occur were censored at the date of the last follow-up. All statistical analyses were performed using IBM SPSS Statistics for Windows ver. 25.0 (IBM Corp., Armonk, NY).
4. Ethical statement
All procedures were conducted in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki declaration. This study was approved by the Institutional Review Board of each hospital (GAIRB2017-365). IRB waived informed consent for this study because of its nature of retrospective analysis.
Discussion
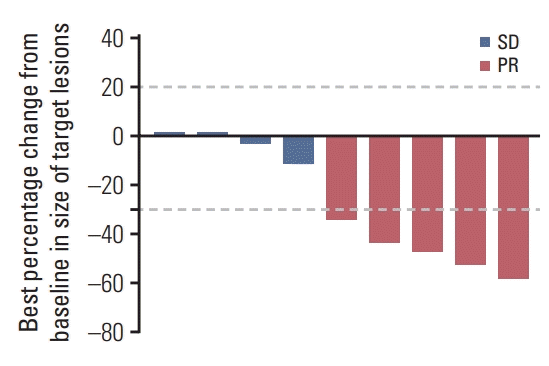
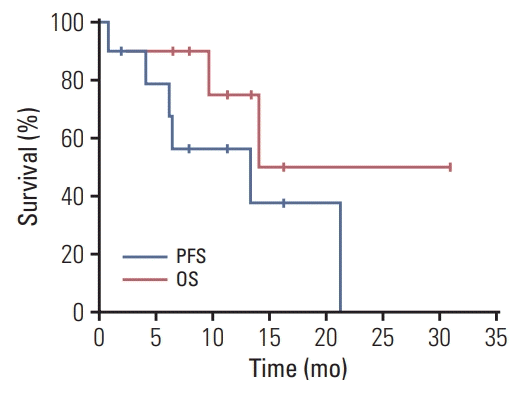
To our knowledge, this is the first report of treatment outcomes not only in Korean patients diagnosed with HLRCC-associated RCC by germline genetic testing but also in realworld practice. We confirmed that the combination of bevacizumab and erlotinib is effective and safe. The ORR was 50% and the median PFS and OS were 13.3 and 14.1 months, respectively. Adverse events were mostly predictable and manageable with routine supportive measures.
HLRCC-associated RCC was only recently categorized by WHO as a distinct entity; therefore, knowledge regarding its clinical features and prognosis is limited. In contrast to other hereditary RCC syndromes, HLRCC-associated RCC is usually unilateral and solitary and metastasis can occur even if primary lesion measures less than 1 cm [
2,
10]. It usually develops in younger people, predominantly between the ages of 15 and 29 years, but can affect patients as young as 11 years [
11]. In our study, the median age at diagnosis was 41 years (range, 27 to 52 years), similar to those in previous reports [
12]. More than half of the patients in the present study had advanced disease at the time of diagnosis. The most common metastatic sites were lymph nodes (abdominal or thoracic), bone, and liver. None of our patients were suspected of having HLRCC-associated RCC by histologic examination. Clinical suspicion is important for a correct diagnosis; thus, physicians may be clued to suspicion by family history, presence of uterine myomas, skin lesions, and clinical course.
Early detection and prompt surgical extirpation can cure the condition, while the prognosis is very poor at advanced stages [
13]. There has been no standard of care for metastatic HLRCC-associated RCC because definitive diagnosis is difficult and its rarity has precluded clinical trials. Thus far, AVATAR is the only clinical trial with promising results for the combination of bevacizumab plus erlotinib. In the interim analysis, the response rates were approximately 29% and 65% for papillary RCC without and with FH mutations, respectively. The median PFS in our group was shorter than that in a previous report (13.3 months vs. 24.2 months) [
8], probably because patients with adverse features were included in our analysis. Furthermore, the small number and frequent censoring of the current study make the survival analysis less reliable.
All patients who received systemic treatment before 2018 started with mTOR inhibitor or VEGFR TKIs, according to clinical practice for non-clear cell RCCs. For these patients, bevacizumab plus erlotinib was administered in later lines. In contrast, four out of five patients diagnosed with metastatic disease in 2018 received bevacizumab and erlotinib as first-line treatment. The reason for the early initiation of bevacizumab plus erlotinib was the physician’s awareness of this entity and guideline recommendation for this treatment. It is not clear whether patients treated with bevacizumab plus erlotinib as an initial treatment showed a better response rate and PFS than those in patients who had received other treatments before bevacizumab plus erlotinib. However, before other promising data are published, bevacizumab and erlotinib should be a standard treatment for HLRCC-associated RCC.
The adverse events of bevacizumab plus erlotinib treatment were mostly manageable. The most common adverse events were acneiform eruption, hypertension, proteinuria, liver function abnormality, and dry skin. Other constitutional symptoms such as fatigue, anorexia, or insomnia also occurred. One patient died of severe intestinal bleeding, which was deemed a complication of the bevacizumab. Therefore, although medical oncologists are familiar with the complications of both erlotinib and bevacizumab, care should be taken to detect potentially life-threatening complications.
In many cancers where targeted therapies like VEGF inhibitor or EGFR inhibitors are frequently used, adverse events attributable to the mechanism of action of molecular targeted agents have been increasingly suggested as predictive biomarker for efficacy [
14,
15]. We explored whether acneiform eruption, hypertension, or proteinuria could be a predictive marker for PFS. Median PFS was 6.5 (95% CI, 0 to 15.5) and 13.4 (95% CI, 0 to 27.4) for patients without and with acneiform eruption, respectively (p=0.36). In addition, median PFS was 6.2 months (95% CI, 1.7 to 10.6) and 21.3 months (95% CI, not calculated) for patients without and with hypertension (p=0.023), and 6.2 months (95% CI, 1.9 to 10.5) and 21.3 months (95% CI not calculated) for patients without and with proteinuria (p=0.029) (
S1 Fig.). To confirm the predictive role of these biomarkers, validation in larger homogeneous prospective cohort (like the AVATAR trial) is needed.
Several prerequisites are essential to improving the clinical practice of HLRCC-associated RCC. Most importantly, physicians should be aware of HLRCC-associated RCC to properly diagnose and treat this disease. Although we reported patients with advanced-stage disease, it is important to detect and cure at early stages. Therefore, genetic counseling and screening of all family members are needed. Furthermore, active surveillance should be performed for early diagnosis of RCC in FH mutation carriers. This strategy has been suggested by the Kidney Cancer Research Network of Canada [
16]. Additionally, the medical costs for genetic testing, surveillance, and treatment of HLRCC are currently not covered by the national health care insurance system in Korea. This financial burden might preclude families with FH mutations from proper and timely treatment and newly-detected families with this rare hereditary cancer syndrome comprise an urgent unmet medical needs in Korea.
This study has several limitations. Above all, this retrospective study included a small number of patients from three academic hospitals. Even though the three hospitals are among the largest in Korea, additional patients may have been diagnosed with HLRCC-associated RCC and received bevacizumab plus erlotinib. Second, there was no central pathology review. However, all participating centers were academic centers possessing genitourinary pathology specialists experienced in RCC. Finally, the PCR for FH was not centralized and was performed in each institution by laboratory-developed tests.
In conclusion, this is the first report on the treatment of advanced HLRCC-associated RCC in real-world practice. Bevacizumab plus erlotinib therapy was effective and the safety profiles were predictable and manageable. Physicians participating in the management of genitourinary cancer should be aware of HLRCC-associated RCC to properly diagnose and treat this disease. Considering its efficacy and safety, bevacizumab plus erlotinib should be the standard of treatment, unless other promising data are published.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download