1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.

2. Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015; 385:977–1010.

3. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011; 365:395–409.

4. Henschke CI, Yankelevitz DF, Mirtcheva R, McGuinness G, McCauley D, Miettinen OS, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol. 2002; 178:1053–7.
5. Nakamura S, Fukui T, Kawaguchi K, Fukumoto K, Hirakawa A, Yokoi K. Does ground glass opacity-dominant feature have a prognostic significance even in clinical T2aN0M0 lung adenocarcinoma? Lung Cancer. 2015; 89:38–42.

6. Ujiie H, Kadota K, Chaft JE, Buitrago D, Sima CS, Lee MC, et al. Solid predominant histologic subtype in resected stage I lung adenocarcinoma is an independent predictor of early, extrathoracic, multisite recurrence and of poor postrecurrence survival. J Clin Oncol. 2015; 33:2877–84.

7. Seok Y, Lee E. Visceral pleural invasion is a significant prognostic factor in patients with partly solid lung adenocarcinoma sized 30 mm or smaller. Thorac Cardiovasc Surg. 2018; 66:150–5.
8. Kadota K, Nitadori J, Sima CS, Ujiie H, Rizk NP, Jones DR, et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol. 2015; 10:806–14.

9. Lu S, Tan KS, Kadota K, Eguchi T, Bains S, Rekhtman N, et al. Spread through Air Spaces (STAS) is an independent predictor of recurrence and lung cancer-specific death in squamous cell carcinoma. J Thorac Oncol. 2017; 12:223–34.

10. Hsu JS, Han IT, Tsai TH, Lin SF, Jaw TS, Liu GC, et al. Pleural tags on CT scans to predict visceral pleural invasion of non-small cell lung cancer that does not abut the pleura. Radiology. 2016; 279:590–6.

11. Ahn SY, Park CM, Jeon YK, Kim H, Lee JH, Hwang EJ, et al. Predictive CT features of visceral pleural invasion by T1-sized peripheral pulmonary adenocarcinomas manifesting as subsolid nodules. AJR Am J Roentgenol. 2017; 209:561–6.

12. Zhao LL, Xie HK, Zhang LP, Zha JY, Zhou FY, Jiang GN, et al. Visceral pleural invasion in lung adenocarcinoma ≤ 3 cm with ground-glass opacity: a clinical, pathological and radiological study. J Thorac Dis. 2016; 8:1788–97.
13. Zwirewich CV, Vedal S, Miller RR, Muller NL. Solitary pulmonary nodule: high-resolution CT and radiologic-pathologic correlation. Radiology. 1991; 179:469–76.

14. Ko JP, Suh J, Ibidapo O, Escalon JG, Li J, Pass H, et al. Lung adenocarcinoma: correlation of quantitative CT findings with pathologic findings. Radiology. 2016; 280:931–9.

15. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016; 11:39–51.
16. Travis WD, Giroux DJ, Chansky K, Crowley J, Asamura H, Brambilla E, et al. The IASLC Lung Cancer Staging Project: proposals for the inclusion of broncho-pulmonary carcinoid tumors in the forthcoming (seventh) edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2008; 3:1213–23.

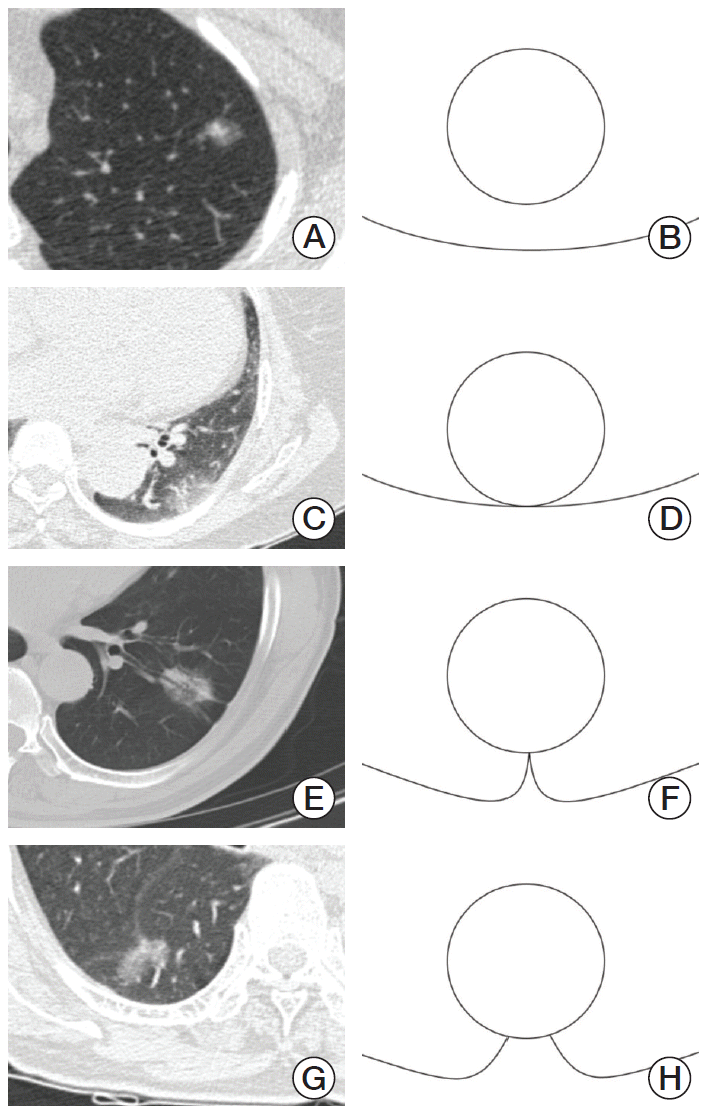
17. Seki N, Fujita Y, Shibakuki R, Seto T, Uematsu K, Eguchi K. Easier understanding of pleural indentation on computed tomography. Intern Med. 2007; 46:2029–30.

18. Shimada Y, Yoshida J, Hishida T, Nishimura M, Ishii G, Nagai K. Predictive factors of pathologically proven noninvasive tumor characteristics in T1aN0M0 peripheral non-small cell lung cancer. Chest. 2012; 141:1003–9.

19. Meniga IN, Tiljak MK, Ivankovic D, Aleric I, Zekan M, Hrabac P, et al. Prognostic value of computed tomography morphologic characteristics in stage I non-small-cell lung cancer. Clin Lung Cancer. 2010; 11:98–104.

20. Zhang H, Lu C, Lu Y, Yu B, Lv F, Zhu Z. The predictive and prognostic values of factors associated with visceral pleural involvement in resected lung adenocarcinomas. Onco Targets Ther. 2016; 9:2337–48.

21. Bak SH, Lee HY, Kim JH, Um SW, Kwon OJ, Han J, et al. Quantitative CT scanning analysis of pure ground-glass opacity nodules predicts further CT scanning change. Chest. 2016; 149:180–91.

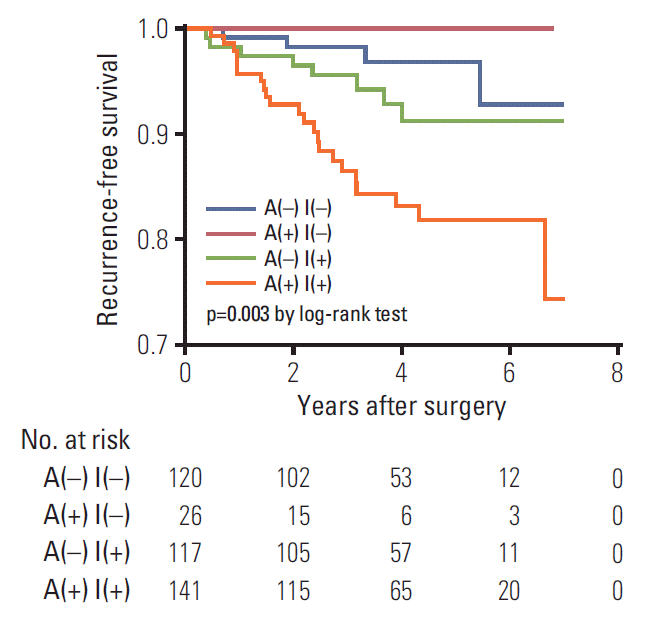
22. Jiang L, Liang W, Shen J, Chen X, Shi X, He J, et al. The impact of visceral pleural invasion in node-negative non-small cell lung cancer: a systematic review and meta-analysis. Chest. 2015; 148:903–11.
23. Huang H, Wang T, Hu B, Pan C. Visceral pleural invasion remains a size-independent prognostic factor in stage I non-small cell lung cancer. Ann Thorac Surg. 2015; 99:1130–9.

24. Liu QX, Deng XF, Zhou D, Li JM, Min JX, Dai JG. Visceral pleural invasion impacts the prognosis of non-small cell lung cancer: a meta-analysis. Eur J Surg Oncol. 2016; 42:1707–13.

25. Hattori A, Suzuki K, Matsunaga T, Takamochi K, Oh S. Visceral pleural invasion is not a significant prognostic factor in patients with a part-solid lung cancer. Ann Thorac Surg. 2014; 98:433–8.
26. Antony VB, Sahn SA, Mossman B, Gail DB, Kalica A. NHLBI workshop summaries. Pleural cell biology in health and disease. Am Rev Respir Dis. 1992; 145:1236–9.
27. Yamamoto T, Kadoya N, Shirata Y, Koto M, Sato K, Matsushita H, et al. Impact of tumor attachment to the pleura measured by a pretreatment CT image on outcome of stage I NSCLC treated with stereotactic body radiotherapy. Radiat Oncol. 2015; 10:35.

28. Wang H, Schabath MB, Liu Y, Berglund AE, Bloom GC, Kim J, et al. Semiquantitative computed tomography characteristics for lung adenocarcinoma and their association with lung cancer survival. Clin Lung Cancer. 2015; 16:e141–63.

29. Naidich DP, Bankier AA, MacMahon H, Schaefer-Prokop CM, Pistolesi M, Goo JM, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013; 266:304–17.

30. Wang T, Zhou C, Zhou Q. Extent of visceral pleural invasion affects prognosis of resected non-small cell lung cancer: a meta-analysis. Sci Rep. 2017; 7:1527.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download