Abstract
Purpose
Treatment targeting immune checkpoint with programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors has demonstrated efficacy and tolerability in the treatment of metastatic urothelial carcinoma (mUC). We investigated the efficacy and safety of atezolizumab in mUC patients who failed platinum-based chemotherapy.
Materials and Methods
A retrospective study using the Samsung Medical Center cancer chemotherapy registry was performed on 50 consecutive patients with mUC treated with atezolizumab, regardless of their PD-L1(SP142) status, as salvage therapy after chemotherapy failure between May 2017 and June 2018. Endpoints included overall response rate (RR), progression-free survival (PFS), and safety.
Results
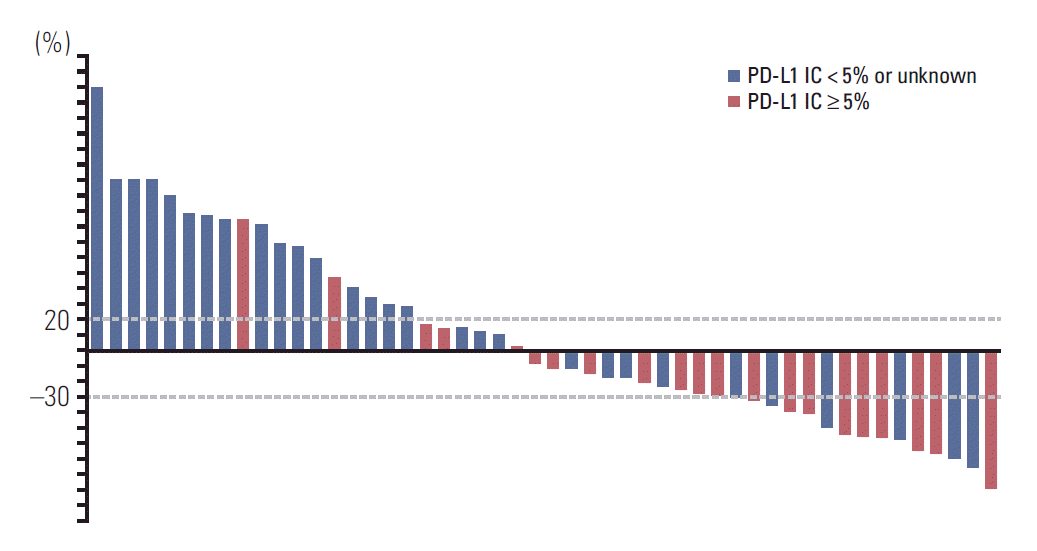
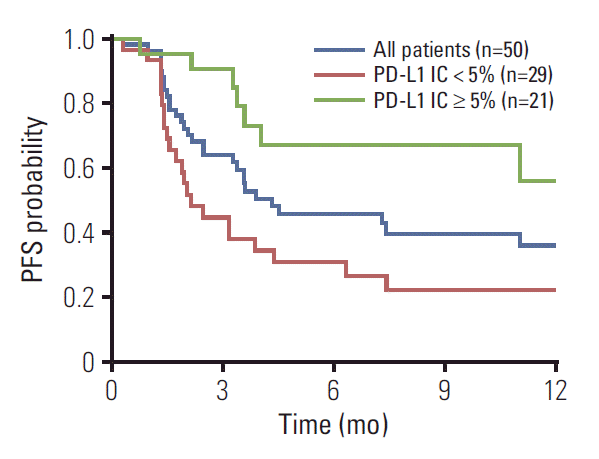
Among 50 patients, men constituted 76% and the median age was 68 years (range, 46 to 82 years). Twenty-three patients (46%) received atezolizumab as second-line therapy. PD-L1 (SP142) status IC0/1 and IC2/3 were found in 21 (42%) and 21 (42%) of patients, respectively; in eight patients (16%), PD-L1 (SP142) expression was not available. Atezolizumab was generally well tolerated, with pruritus and fatigue being the most commonly observed toxicities. As a result, partial response was noted in 20 patients (40%), with 12 (24%) stable diseases. RRwas higherin IC2/3 (62%) than in IC0/1 patients (24%, p=0.013). The median PFS was 7.4 months (95% confidence interval, 3.4 to 11.4 months). As expected, PFS also was significantly longer in IC2/3 patients than in IC0/1 (median, 12.7 vs. 2.1 months; p=0.005). PFS was not significantly influenced by age, sex, performance status, number of previous chemotherapy, site of metastases, or any of the baseline laboratory parameters.
Go to : 
Bladder cancer, the seventh most common malignancy in Korea [1], is the most frequent among urothelial carcinoma (UC) that also include the less common UC arising from renal pelvis, ureter and urethra. Over the past two decades, there has been no significant improvement in survival of UC with 5-year survival rates for locally-advanced and metastatic disease of 33% and 5%, respectively [2]. In metastatic UC (mUC), platinum-based combination chemotherapy has been the standard-of-care with a median overall survival (OS) of approximately 15 months [3]. Recently, programmed death-1 (PD-1) and programmed death-ligand 1 (PD-L1) checkpoint inhibitors including atezolizumab and pembrolizumab received approval for the treatment of patients with mUC who have disease progression during or following platinum-based chemotherapy, regardless of PD-L1 expression level [4,5]. In Korea, although reimbursement is provided only for patients with PD-L1 positive disease since the approval in May 2017, atezolizumab has been widely administered to mUC patients pretreated with platinum-based chemotherapy.
Despite promising clinical data with immune checkpoint inhibitors, not all patients with mUC who are treated with atezolizumab achieve clinical response, and even in the responders, resistance to therapy will, if not all, eventually develops. Considering most mUC patients would receive platinum-based chemotherapy in first-line setting, and in an effort to generate real-world data in Korean mUC patients, we conducted a retrospective review of a prospectively collected cancer chemotherapy registry. Herein, we report the outcomes of mUC patients who received atezolizumab following clinical failure to platinum-based chemotherapy. Although this study is limited by the retrospective nature of the analysis, the present evaluation was also done with the intent to develop improved therapeutic strategies for pretreated mUC patients and support further prospective studies to better define the full therapeutic potential of immune checkpoint inhibitors.
Go to : 
All patients, with histologically-proven mUC, had been treated with platinum-based combination chemotherapy for metastatic disease. With the help of Samsung Medical Center (SMC) cancer registry, we retrospectively collected and reviewed follow-up data of adult mUC patients (> 20 years) who were consecutively treated with atezolizumab as salvage therapy, between May 2017 and June 2018. Other criteria for case inclusion were as follows: (1) histologically confirmed diagnosis of UC arising from bladder and/or upper urinary tract, (2) presence of measurable metastatic disease, and (3) availability of clinical data at the beginning of therapy and follow-up. We excluded patients who were enrolled in clinical trials to ensure the study population reflected our daily clinical practice, and the choice of atezolizumab was solely at the discretion of the treating oncologists.
In all patients, archival tumor samples were evaluated with SP142 PD-L1 immunohistochemical assay (Ventana, Tucson, AZ). According to the current Korean Health Insurance policy, only patients with PD-L1 expression on 5% or more of tumor-infiltrating immune cells (IC2/3) were reimbursed for atezolizumab. For patients with PD-L1 (SP142) IC0 or IC1, as well as those with no available PD-L1 data, atezolizumab was self-paid in full. Atezolizumab 1,200 mg was administered intravenously every 3 weeks. Supportive care including the administration of blood products, palliative radiotherapy for painful bone metastases, and the use of analgesics was given if judged appropriately by the treating physicians. Before initiating the first dose of atezolizumab, patients had a complete history taken, complete blood counts and serum chemistries, chest X-rays, and computed tomography (CT) scans of all involved sites. Baseline characteristics and outcome data were collected using a uniform case report form. In order to evaluate clinical response to atezolizumab, CT scans were usually performed every 6 weeks. Response Evaluation Criteria in Solid Tumors (RECIST v1.1) and the assessment of the treating physicians were used to categorize response. Atezolizumab was continued until objective disease progression, unacceptable toxicity, or patient’s refusal.
Primary endpoint of the present study was the overall response rate (RR). Secondary endpoints included progression-free survival (PFS) and safety. Time from the first day of atezolizumab administration to the date of documented disease progression or death was used to calculate PFS. PFS was calculated using the Kaplan-Meier method. To examine the impact of baseline parameters collected on PFS, Cox proportional hazard model was used. The potential presence of interaction effects between baseline parameters was tested by defining product terms for the respective factors in a regression model. All p-values were two-sided, with p < 0.05 indicating statistical significance. All analyses were performed using the R for Windows v2.11.1 software (R Core Team, Vienna, Austria; http://www.r-project.org).
This retrospective study was approved by the Institutional Review Board of SMC (approval number: SMC 2018-02-016) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was waived for this study, because of its retrospective nature.
Go to : 
Medical records from 50 eligible patients who were consecutively treated with atezolizumab for pretreated mUC at the medical oncology department of SMC between May 2017 and June 2018 were collected for the present retrospective study. Patient characteristics are given in Table 1. As shown, men constituted 76% of the patients. All patients had previously been treated with gemcitabine plus platinum combination chemotherapy, and 46% received atezolizumab as second-line therapy. Among 42 patients whose PD-L1 expression was available, 21 (50%) had IC2/3 (i.e., 5% or higher). Approximately half of the patients had two or more metastatic disease sites, mostly involving lung, bone and lymph nodes. At the time of data collection, with a median follow-up duration of 14 months, 33 patients (66%) discontinued atezolizumab and 32 patients (64%) had experienced disease progression.
Patient characteristics
Patients received atezolizumab for a total of 318 cycles (median, 5; range, 1 to 17). The most common reason for therapy discontinuation was disease progression (58%), followed by toxicity (4%) and economic burden (4%). Overall, salvage atezolizumab was generally well tolerated, with fatigue and pruritus being the most commonly observed toxicities (Table 2). Severe and persistent immune-related adverse events resulting in treatment discontinuation were observed in two patients. Three patients (6%) had an immune-related adverse event that necessitated systemic corticosteroid use. One patient died of causes whose relation to atezolizumab could not be completely ruled out (68-year-old male, who was known to have multiple lung and pleural metastases, died of respiratory failure shortly after the first dose of atezolizumab therapy). Although the extent of pleural effusions and pneumonitis was markedly increased, the possibility of drug-related mortality was not completely excluded.
Most commonly observed adverse events per patients (n=50)
Response evaluation was possible in 50 patients. In an intent-to-treat analysis, partial responses to atezolizumab were noted in 20 patients (RR, 40%; 95% confidence interval [CI], 26 to 54). Stable disease was observed in 12 patients (24%), leading to a 64% disease control rate. Except for PD-L1 status (Fig. 1), RR was not significantly influenced by age, sex, performance status, primary site, number of prior chemotherapy regimens, or number and site of metastases. We observed higher RR in patients with PD-L1 IC2/3 (62%) than in those with PD-L1 IC0/1 (62% vs. 24%, p=0.013). Of the 50 patients analyzed in the study, the median PFS was 7.4 months (95% CI, 3.4 to 11.4) (Fig. 2). The estimated PFS was significantly longer for patients with PD-L1 IC2/3 (median, 12.7 months; 95% CI, 10.5 to 14.8) compared to those with PD-L1 IC0/1/unknown (median, 2.1 months; 95% CI, 1.6 to 2.6; log-rank p=0.005). In a Cox proportional hazards model, only PD-L1 IC2/3 was associated with the lower risk for disease progression (hazard ratio, 0.26; 95% CI, 0.10 to 0.66).
For exploratory purpose, we compared PFS according to the clinical response to atezolizumab. PFS was longer (p < 0.001) in patients who achieved response (median, 12.8 months) than in non-responders (2.1 months). After atezolizumab failure, 32% of the patients received third-line therapy, mostly with taxanes (n=11) or investigational agents (n=6).
Go to : 
This retrospective study was designed to evaluate the activity and safety of salvage atezolizumab therapy in a subset of Korean patients with mUC who had been treated with platinum-based combination chemotherapy. The 40% RR, together with an additional stable disease rate of 24%, provided an overall disease control rate of 64%. A median PFS of 7.4 months in PD-L1 unselected patients compared favorably with the results observed in the pivotal phase 2/3 studies [4,6], as well as those seen in other second-line mUC studies involving pembrolizumab [5], nivolumab [7], durvalumab [8], or avelumab [9]. PFS and overall response may be good because more IC2/3 patients (n=21, 50%) enrolls in this study than other studies.
In metastatic or advanced setting, platinum-based combination chemotherapy has been regarded as standard treatment, because it has shown remarkable RR and PFS, as well as tolerability [3]. However, most patients would develop resistance to these chemotherapy regimens after months, or even after years, of clinical benefit. In these patients, despite the lack of evidence for benefit associated with patients may benefit from second-line treatment, it is common practice to offer further chemotherapy involving taxanes, pemetrexed, or other platinum-based regimens. Since currently another immune checkpoint inhibitor pembrolizumab [5] had demonstrated activity when compared with chemotherapy, the optimal second-line therapy in patients with mUC is still undecided. While pembrolizumab was not readily available as second-line therapy when the present study was initiated, selection based on the benefits of atezolizumab versus pembrolizumab should come from randomized, direct comparison clinical trials.
Surprisingly, phase 3 trials involving pembrolizumab (KEYNOTE-045) and atezolizumab (IMvigor-211) revealed different results and conclusions [5,6]. In KEYNOTE-045, where pembrolizumab was compared with chemotherapy [5], significantly longer OS (10.3 months vs. 7.4 months) and RR (21% vs. 11%) were demonstrated. It was of note that the benefit of pembrolizumab over chemotherapy was seen in both the total population and those with a PD-L1 overexpression. In IMvigor-211 [6], unfortunately, atezolizumab was not associated with longer OS than chemotherapy in total population (8.6 months vs. 8.0 months) or patients with PD-L1 IC2/3 mUC (11.1 months vs. 10.6 months). Nevertheless, although the efficacy of second-line atezolizumab was not evident in the phase 3 IMvigor-211 trial, the authors concluded that the well-tolerated and durable remissions observed with atezolizumab can be considered favorable for patients with mUC previously treated with platinum-based chemotherapy. Everyone knows that indirect comparisons of different randomized trials should be based on similarity and consistency assumptions but often lead to bias. Currently, the lesson we learned from these two trials is that immune checkpoint inhibitors targeting PD-1/PD-L1 offer clinically relevant benefit in patients with platinum-treated mUC.
Therefore, the identification of prognostic or predictive factors allowing the selection of patients who are likely to benefit from PD-1/PD-L1 inhibitors is an important challenge. Our study showed that, among the total of 50 patients with mUC, 18 patients (36%) experienced a progressive disease as their best response to atezolizumab, comparable to findings in published reports [4,6]. The patients have been treated outside the clinical trials, thus accurately reflecting the current clinical practice in mUC. Although this study is retrospective in nature, it is clear that atezolizumab may not be beneficial for all mUC patients with chemotherapy failure. One of the most widely recognized predictive markers for immune checkpoint inhibitors is PD-L1 expression [10]. In the present study, the difference in RR (62% vs. 24%) and PFS (12.7 months vs. 2.1 months) between patients with PD-L1 IC0/1 and IC2/3 was distinct. However, the presence of robust responses in some patients with IC0/1, as well as the similar outcomes seen in phase 3 studies, complicates the issue of PD-L1 as an exclusionary predictive biomarker. Tumor mutation burden, which is known to be high in UC, and DNA mismatch repair deficiency (dMMR) are thought to be surrogate markers for benefit from immune checkpoint inhibitor therapy [10]. Tumor mutation burden, dMMR and PD-L1 expression are somewhat interrelated [11], as mechanistically the relationship between these biomarkers may be related to increased neoantigen load required for immune recognition of tumors. Although we found that response to atezolizumab was significantly related to the PD-L1 status, it should be noted that the present retrospective study may have a selection bias. Actually, among the immune checkpoint inhibitors approved for salvage treatment of mUC, only atezolizumab is fully reimbursed by the health insurance system in Korea. Since the reimbursement is provided only for patients with PD-L1 IC2/3 disease, and our patients received atezolizumab at the discretion of the treating medical oncologists, it may be that clinical and/or economic judgment withheld the use of atezolizumab from those with first-line chemotherapy failure. Therefore, we still do not know whether the PD-L1 indicates optimal forms of treatment for the individual patient. Needless to say, when interpreting the results, it is of note that this analysis represents only a small sample of patient, but it is possible that patients with PD-L1 IC0/1 disease may have more aggressive disease than those with IC2/3. In recent years, extensive translational and clinical researches are underway to identify biologically relevant biomarkers for PD-1/PD-L1 therapy. Since immunotherapy with intravesical bacillus Calmette-Guerin was the first effective treatment in UC [12], immune checkpoint inhibitors, alone or in combination with chemotherapy, are in development for UC in earlier stage of disease with promising results [13,14].
In conclusion, the present study demonstrates that salvage atezolizumab platinum-based combination chemotherapy provides clinically relevant efficacy and tolerability in patients with mUC. It is suggested that the magnitude of the benefit of atezolizumab in Korean patients are similar to that obtained in the phase 2/3 trials. Although not confirmed in the prospective IMvigor-211 trial, we found that patients with PD-L1 IC2/3 tumors had better clinical outcomes that those in IC0/1 tumors. It is hoped that, with better patient selection, clinical outcomes of mUC patients can be improved. Furthermore, emerging science and the knowledge of the disease may further guide us to develop individualized treatment for UC patients.
Go to : 
References
1. Roscigno M, Shariat SF, Freschi M, Margulis V, Karakiewizc P, Suardi N, et al. Assessment of the minimum number of lymph nodes needed to detect lymph node invasion at radical nephroureterectomy in patients with upper tract urothelial cancer. Urology. 2009; 74:1070–4.

3. von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005; 23:4602–8.

4. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016; 387:1909–20.
5. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017; 376:1015–26.

6. Powles T, Duran I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018; 391:748–57.

7. Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017; 18:312–22.

8. Powles T, O'Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017; 3:e172411.
9. Patel MR, Ellerton J, Infante JR, Agrawal M, Gordon M, Aljumaily R, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018; 19:51–64.

10. Kim ST, Klempner SJ, Park SH, Park JO, Park YS, Lim HY, et al. Correlating programmed death ligand 1 (PD-L1) expression, mismatch repair deficiency, and outcomes across tumor types: implications for immunotherapy. Oncotarget. 2017; 8:77415–23.

11. Salem ME, Puccini A, Grothey A, Raghavan D, Goldberg RM, Xiu J, et al. Landscape of tumor mutation load, mismatch repair deficiency, and PD-L1 expression in a large patient cohort of gastrointestinal cancers. Mol Cancer Res. 2018; 16:805–12.

12. Lamm DL, Thor DE, Stogdill VD, Radwin HM. Bladder cancer immunotherapy. J Urol. 1982; 128:931–5.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download