Introduction
Materials and Methods
1. Case selection
2. Data collection
3. Statistical analysis
4. Ethical statement
Results
1. Clinical characteristics
Table 1.
| Case No. | Sex | Age at diagnosis (yr) | Site of lymphoma involvement at diagnosis | HHV8 | ECOG PS | IPI score | Comorbidity | Treatmenta) | Response to chemotherapy | PFS (mo) | OS (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 70 | Pleural cavity | Positive | 1 | 1 | Lung cancer | 1st-line CHOP | PR | ≥ 6 | ≥ 11 |
| 2 | M | 67 | Pleural cavity, bone marrow, cervical lymph node | Positive | 1 | 4 | HBV | 1st-line CHOP | NA | ≥ 6 | ≥ 9 |
| 3 | M | 59 | Pleural cavity | Positive | 1 | 1 | HIV | No chemotherapy | NA | ≥ 4 | ≥ 14 |
| 4 | F | 78 | Pleural cavity | Positive | 2 | 4 | None | No chemotherapy | NA | 49 | 49 |
| 5 | M | 87 | Pleural cavity | Positive | 3 | NA | None | No chemotherapy | NA | ≥ 5 | ≥ 6 |
| 6 | M | 60 | Pleural cavity, pericardial cavity, peritoneal cavity | Positive | 2 | 3 | None | 1st-line CHOP | CR followed by PD | 28 | ≥ 173 |
| 2nd-line ICE → autoHSCT | PR | ||||||||||
| 7 | F | 59 | Vitreous body (eyeball) | Negative | 0 | 0 | HCV | 1st-line HD-MTX | CR | 66 | 66 |
| 8 | M | 80 | Pleural cavity, pericardial cavity | Negative | 3 | 5 | None | 1st-line R-CHOP | CR | ≥ 17 | ≥ 17 |
| 9 | M | 70 | Pericardial cavity | Negative | 1 | 3 | None | 1st-line R-CHOP | CR | ≥ 14 | ≥ 14 |
| 10 | M | 77 | Pleural cavity, peritoneal cavity | Negative | 2 | 5 | None | 1st-line R-CHOP | CR | ≥ 12 | ≥ 12 |
| 11 | M | 83 | Pleural cavity | Negative | 2 | 4 | None | 1st-line R-CVP | NA | ≥ 2 | ≥ 2 |
| 12 | M | 75 | Pleural cavity, pericardial cavity | Negative | 1 | 3 | None | 1st-line R-CHOP | CR | ≥ 46 | ≥ 49 |
| 13 | F | 76 | Pleural cavity | Unknown | 2 | 2 | None | 1st-line CHOP | CR | ≥ 5 | ≥ 5 |
| 14 | F | 73 | Pleural cavity, peritoneal cavity | Unknown | 1 | 4 | HBV | 1st-line CVP | PR | 10 | 10 |
| 15 | M | 86 | Pleural cavity | Unknown | 1 | 4 | None | 1st-line R-CVP | PR | ≥ 24 | ≥ 24 |
| 16 | F | 66 | Pleural cavity, peritoneal cavity | Unknown | 1 | 4 | None | 1st-line CHOP | CR | ≥ 99 | ≥ 99 |
| 17 | F | 39 | Pleural cavity, lung | Unknown | 1 | 3 | None | 1st-line R-CHOP | CR | ≥ 123 | ≥ 123 |
HHV8, human herpesvirus 8; ECOG PS, Eastern Cooperative Oncology Group performance status; IPI, international prognostic index; PFS, progression-free survival; OS, overall survival; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisolone; PR, partial remission; HBV, hepatitis B virus; NA, not assessed or not available; HIV, human immunodeficiency virus; ICE, ifosfamide, carboplatin, and etoposide; autoHSCT, autologous hematopoietic stem cell transplantation; CR, complete remission; PD, progressive disease; HCV, hepatitis C virus; HD-MTX, high-dose methotrexate; R-CHOP, rituximab plus CHOP; CVP, cyclophosphamide, vincristine, and prednisolone; R-CVP, rituximab plus CVP.
Table 2.
| Characteristic | Entire cohort (n=17) | PEL (n=6) | HHV8-unrelated BCBL (n=6) | HHV8-unknown BCBL (n=5) |
|---|---|---|---|---|
| Age at diagnosis (yr) | 73 (39-87) | 68.5 (59-87) | 76 (59-83) | 73 (39-86) |
| Sex | ||||
| Male | 11/17 (64.7) | 5/6 (83.3) | 5/6 (83.3) | 1/5 (20.0) |
| Female | 6/17 (35.3) | 1/6 (16.7) | 1/6 (16.7) | 4/5 (80.0) |
| Site of lymphoma involvement | ||||
| Pleural cavity | 15/17 (88.2) | 6/6 (100) | 4/6 (66.7) | 5/5 (100) |
| Pericardial cavity | 4/17 (23.5) | 1/6 (16.7) | 3/6 (50.0) | 0/5 (0) |
| Peritoneal cavity | 4/17 (23.5) | 1/6 (16.7) | 1/6 (16.7) | 2/5 (40.0) |
| Extra-cavitary lesionb) | 2/17 (11.8) | 1/6 (16.7) | 0/6 (0) | 1/5 (20.0) |
| ECOG PS at diagnosis | ||||
| 0-1 | 10/17 (58.8) | 3/6 (50.0) | 3/6 (50.0) | 4/5 (80.0) |
| 2-4 | 7/17 (41.2) | 3/6 (50.0) | 3/6 (50.0) | 1/5 (20.0) |
| IPI risk group | ||||
| Low (0-1) | 3/16 (18.8) | 2/5 (40.0) | 1/6 (16.7) | 0/5 (0) |
| Low-intermediate (2) | 1/16 (6.2) | 0/5 (0) | 0/6 (0) | 1/5 (20.0) |
| High-intermediate (3) | 4/16 (25.0) | 1/5 (20.0) | 2/6 (33.3) | 1/5 (20.0) |
| High (4-5) | 8/16 (50.0) | 2/5 (40.0) | 3/6 (50.0) | 3/5 (60.0) |
| Comorbidity | ||||
| HIV | 1/17 (5.9) | 1/6 (16.7) | 0/6 (0) | 0/5 (0) |
| HBV | 2/17 (11.8) | 1/6 (16.7) | 0/6 (0) | 1/5 (20.0) |
| HCV | 1/17 (5.9) | 0/6 (0) | 1/6 (16.7) | 0/5 (0) |
| Co-occurring malignancy | 1/17 (5.9)c) | 1/6 (16.7)c) | 0/6 (0) | 0/5 (0) |
| Laboratory profile at diagnosis | ||||
| WBC ≥ 10,000/μL | 3/17 (17.6) | 1/6 (16.7) | 0/6 (0) | 2/5 (40.0) |
| Hemoglobin < 10 g/dL | 4/17 (23.5) | 1/6 (16.7) | 2/6 (33.3) | 1/5 (20.0) |
| Platelet < (130×103)/μL | 1/17 (5.9) | 1/6 (16.7) | 0/6 (0) | 0/5 (0) |
| Serum albumin < 3.5 g/dL | 12/17 (70.6) | 5/6 (83.3) | 5/6 (83.3) | 2/5 (40.0) |
| Serum creatinine ≥ 1.5 mg/dL | 3/17 (17.6) | 1/6 (16.7) | 1/6 (16.7) | 1/5 (20.0) |
| LDH above normal | 13/16 (81.2) | 4/6 (66.7) | 4/5 (80.0) | 5/5 (100) |
| β2-microglobulin ≥ 3.5 mg/dL | 5/10 (50.0) | 2/4 (50.0) | 2/5 (40.0) | 1/1 (100) |
| C-reactive protein ≥ 0.8 mg/dL | 17/17 (100) | 6/6 (100) | 6/6 (100) | 5/5 (100) |
Values are presented as median (range) or number (%). HHV8, human herpesvirus 8; PEL, primary effusion lymphoma; BCBL, body cavity-based lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; IPI, international prognostic index; HIV, human immunodeficiency virus; HBV, hepatitis B virus; HCV, hepatitis C virus; WBC, white blood cell; LDH, lactate dehydrogenase.
a) The proportions are calculated as the frequency divided by the total number of evaluable patients for each characteristic,
2. Immunophenotype and EBV status
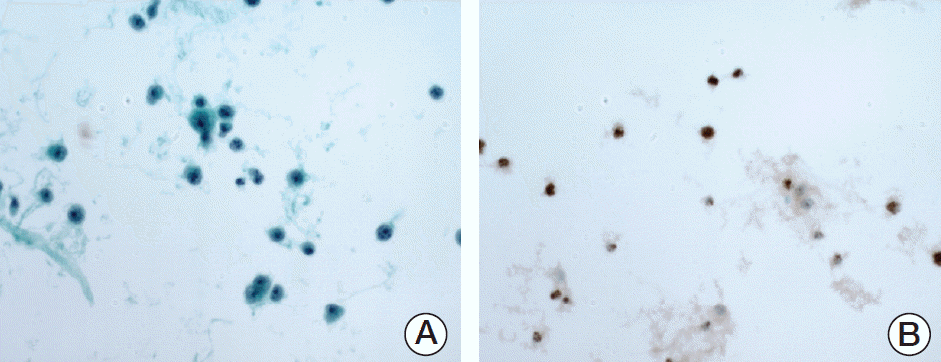 | Fig. 1.Lymphoma cells with positive nuclear staining for latency-associated nuclear antigen 1 in the ascitic fluid of a representative primary effusion lymphoma patient. (A) Ascites smear exhibits individually scattered tumor cells with an immunoblastic or plasmablastic cytomorphology. (B) Immunohistochemistry using an anti‒human herpesvirus 8 antibody shows dark brown nuclear staining of tumor cells. |
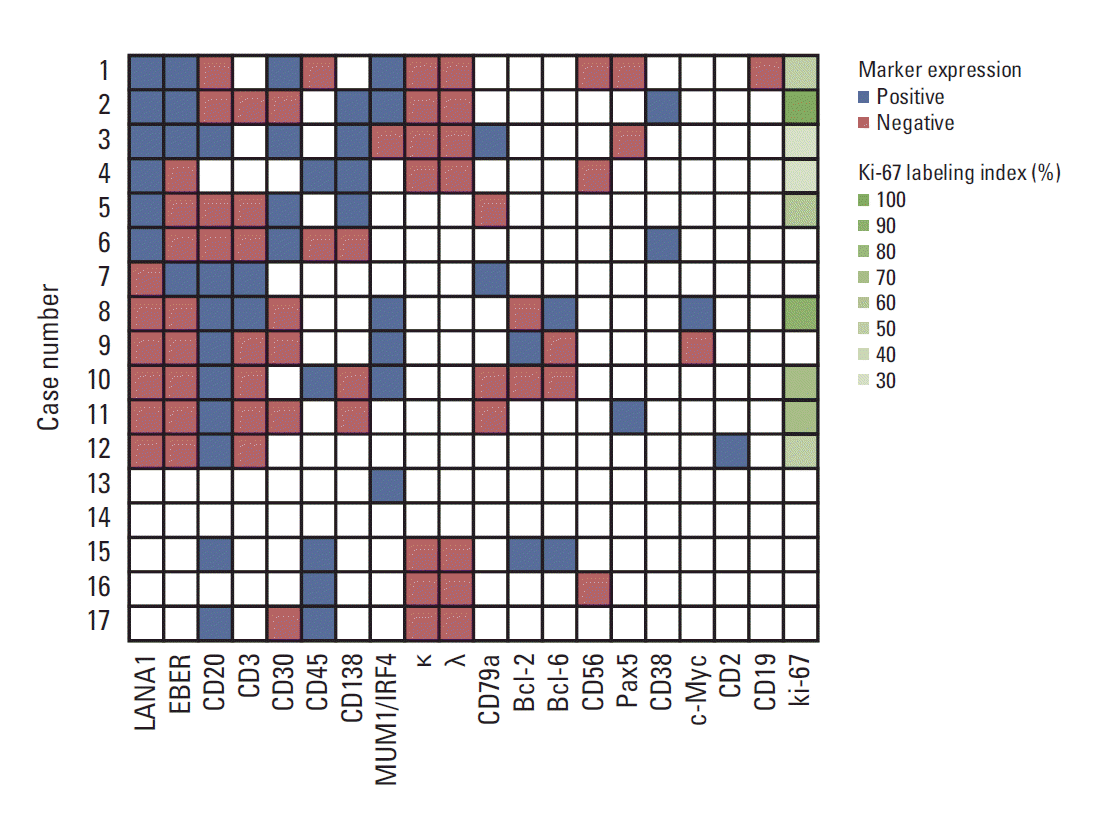 | Fig. 2.Immunophenotypic analysis and in situ hybridization for Epstein-Barr virus–encoded small RNA (EBER). The cases are presented in the same order as in Table 1. Latency-associated nuclear antigen 1 (LANA1) and EBER are shown in the first and second columns, respectively, while the remaining markers are arranged in descending order of the number of cases in which they were analyzed. All markers that were stained in at least one case are shown. Blank tiles indicate that assays were not performed or data were not available. MUM1, multiple myeloma oncogene 1; IRF4, interferon regulatory factor 4; κ, immunoglobulin κ light chain; λ, immunoglobulin λ light chain. |
3. Treatment and response
Table 3.
Values are presented as number (%). PEL, primary effusion lymphoma; HHV8, human herpesvirus 8; BCBL, body cavity-based lymphoma; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisolone; R-CHOP, rituximab plus CHOP; CVP, cyclophosphamide, vincristine, and prednisolone; R-CVP, rituximab plus CVP; HD-MTX, high-dose methotrexate.
4. Survival
Discussion
Table 4.
| Characteristic | Nador et al. [4] | Boulanger et al. [5] | Simonelli et al. [6] | Boulanger et al. [7] | Guillet et al. [8] | Present cohort |
|---|---|---|---|---|---|---|
| No. of cases | 15 | 12 | 11 | 28 | 34 | 6 |
| Male-to-female ratio | 15:0 | 12:0 | 10:1 | 27:1 | 31:3 | 5:1 |
| Age, median (range, yr) | 44 (31-85) | 43.5 (33-66) | 41 (26-58) | 44 (33-78) | 45 (40-54) | 68.5 (59-87) |
| HIV positivity rate (%) | 87 | 100 | 100 | 100 | 100 | 17 |
| Median survival | 5 mo | 5.6 mo | 6 mo | 6.2 mo | 10.2 mo | 4.1 yr |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download