Abstract
Purpose
This study was aimed to investigate the role of poly(A)-binding protein-interacting protein 1 (Paip1) in cervical carcinogenesis.
Materials and Methods
The expression of Paip1 in normal cervical epithelial tissues and cervical cancer (CC) tissues were detected by immunohistochemistry. In vivo and in vitro assays were performed to validate effect of Paip1 on CC progression.
Results
Paip1 was found to be up-regulated in CC, which was linked with shorter survival. Knockdown of Paip1 inhibited cell growth, induced apoptosis and cell cycle arrest in CC cells, whereas its overexpression reversed these effects. The in vivo tumor model confirmed the pro-tumor role of Paip1 in CC growth.
Cervical cancer (CC) is the fourth most common cause of cancer in women worldwide [1-3]. With the increasing uptake of Pap smear screening and hepatitis B virus vaccines in recent years, the incidence of CC has been decreased in many countries [4,5]. However, there are still approximately 527,600 new cases and 265,700 deaths worldwide in 2012. Especially in developing countries, CC ranks as the second most commonly diagnosed cancer and the third leading cause of cancer death [6]. Unfortunately, there are no definite diagnostic and prognostic biomarkers [7].
Poly(A)-binding protein-interacting protein 1 (Paip1) is a 479-residue protein which encoded by PAIP1. Paip1 was involved in the initiation of translation in eukaryotes by binding polyadenylate-binding protein cytoplasmic 1 and several eukaryotic initiation factors 4F complexes [8,9]. Paip1 stimulated translation rates and therefore implicated in several pathogenic roles [10,11]. Scotto et al. [12] mentioned that Paip1 was up-regulated in invasive CCs and played a role in DNA repair and cell cycle regulation. Subsequently, Piao et al. [13] reported that Paip1 was found to be overexpressed in breast cancer, and its expression was closely related to cell cycle and poor prognosis. These results indicated that targeting Paip1 might be an effective anti-tumor therapy strategy. However, the biological role and related mechanisms of Paip1 in CC progression have not been studied.
Therefore, our data examined the relationship between Paip1 overexpression and clinicopathological features of CC and assessed its prognostic value. We also observed that Paip1 promoted CC cell proliferation, arrested cell cycle progression and inhibited cell apoptosis. These studies have promoted our understanding of the role of Paip1 in CC development and its related molecular mechanisms.
Antibodies against Paip1, cyclin B1, CDK1, P21, Bax, Bcl2, and actin were purchased from Proteintech Technology (Wuhan, China). Antibody against Ki-67 was purchased from BOSTER (Wuhan, China).
Routinely diagnosed primary CC tissues (130 cases: squamous cervical cancers, 121; adenocarcinomas, 9), cervical intraepithelial neoplasia (CIN) tissues (CIN-1, 22; CIN-2, 34; CIN-3, 38) and normal cervical epithelial tissues (47 cases) with clinical features were collected in the present study. All tissue specimens were taken from patients who underwent CC surgery and who did not receive any preoperative tumor therapy. Diagnosis and staging were based on the Staging Manual of the American Joint Committee on Cancer, 7th edition. Clinical information on the samples is summarized in Tables 1 and 2.
The human CC cell lines (SiHa, HeLa, C33A, and Caski) and normal non-malignant breast cells (MCF-10A) (American Type Culture Collection, Manassas, VA) were cultured in Dulbecco's modified Eagle medium containing 10% fetal bovine serum and streptomycin-penicillin (100 U/mL). Cells were incubated at 37°C in an atmosphere of 5% CO2.
Stable knockdown of Paip1 was generated by transducing the sh-Paip1 lentiviral expression construct in cells (Genechem, Shanghai, China). The shRNA sequence used was the following: 5′-GCTTCTATGCC TACAACTT-3′. Stable lentiviral transfected SiHa and HeLa were selected using 2 μg/mL puromycin for 2 weeks. Transfection efficiency was confirmed by western blot. Human Paip1 cDNA was purchased form (Genechem) and cloned into the GV144 plasmid. The Paip1 plasmid and corresponding empty vector were transfected into CC cell (C33A) using Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s protocol.
Western blot and protein detection was performed as previously described [14]. The harvested cells were prepared with RIPA cell lysis buffer containing a protease inhibitor mixture, and then run on sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Immobilon P, Millipore, Bedford, MA), probed with primary antibodies and second antibodies. At last, the results were analyzed quantitatively using chemiluminescent and fluorescent imaging system.
Cells were plated into 96-well plates and cultured with indicated compounds for 0, 24, 48, and 72 hours, followed by the addition of 100 μL of MTT solution to each well and further incubation for 4 hours. Removed the medium from the wells and added 100 μL dimethyl sulfoxide into each well. The relative number of surviving cells was assessed by measuring the absorbance at 570 nm.
Cells were plated on 6-well plates at a concentration of 5,000 cells for each well and the medium was replaced every 3 days. Cells were fixed and stained with 1% crystal violet after incubating for 2 weeks.
Cell cycle status was determined by measuring cellular DNA content after staining with propidium iodide (PI; BD Bioscience, San Jose, CA). Briefly, cells in the log growth phase were seeded at a density of 2×105 cells per well in 6-well plates. After treatment, cells were harvested and fixed in 70% cold ethanol at –20°C overnight. The next day, cells were treated with 100 μg/mL of RNase and incubated at 37°C for 30 minutes. Then, cells stained with 100 μg/mL of PI in the dark at room temperature, for another 30 minutes. Samples were subsequently analyzed with the FACScalibur flow cytometer (BD Bioscience), and data were analyzed with ModFit LT software (Verity Software House, Topsham, ME).
Apoptosis was evaluated in CC cells with Annexin V-FITC and PI staining (20 minutes; BD Biosciences). Cells were analyzed using a flow cytometer (BD Biosciences) according to the manufacturer’s instructions [15].
BALB/c nude mice were housed in specific pathogen-free conditions following the guidelines of the Institutional Animal Care. To assess the effect of Paip1 on tumorigenicity in vivo, CC cells were subcutaneously injected into the left or right flank of the mice. Animals were sacrificed after 6 weeks of treatment, and then the mean tumor weight was measured. Tumor sizes were measured every 7 days.
For Immunohistochemical (IHC) studies with a DAKO LSAB kit (DAKO A/S, Glostrup, Denmark), tissue sections were deparaffinized, rehydrated and incubated with 3% H2O2 in methanol for 15 minutes at room temperature (RT) to eliminate endogenous peroxidase activity. Antigen retrieval was performed by placing the slides in 0.01 M sodium citrate buffer (pH 6.0) at 95°C for 20 minutes. The slides were then incubated with the primary antibody at 4°C overnight. After incubation with the secondary antibody at RT for 1 hour, immunostaining was developed using DAB, and the slides were counterstained with hematoxylin [16].
Statistics of IHC scores were conducted according to the ratio and intensity of positive-staining areas. Staining areas were scored as ‘0’ (negative, no or less than 5% positive cells), ‘1’ (6%-25% positive cells), ‘2’ (26%-50% positive cells), ‘3’ (50%-75% positive cells), and ‘4’ (more than 75% positive cells). And the staining intensities were scored as ‘0’ (no staining), ‘1’ (weak staining), ‘2’ (moderate staining), and ‘3’ (intense staining). A final score was calculated by multiplying these two parameters. All slides were scored independently by two pathologists, who were blind to all clinical data.
The data analysis was performed using SPSS ver. 17.0 software (SPSS Inc., Chicago, IL) and GraphPad Prism 6.0 software (GraphPad Software Inc., San Diego, CA). Group comparisons for continuous data were done by t test or oneway ANOVA. Biochemical experiments were performed in triplicate and a minimum of three independent experiments were evaluated. The value of p < 0.05 was considered statistically significant.
This study complied with the principles of the Declaration of Helsinki and was approved by the human ethics and research ethics committees of Yanbian University Medical College in China. The resected specimens from patients who underwent surgery were potentially used for scientific researches and publication of identifying information/ images (when applicable). Their privacy will be maintained. The follow-up survival data were collected retrospectively through medical-record analyses.
All experiments were performed in keeping with the procedures and protocols of the Animal Ethics Committee of Yanbian University.
The Oncomine data sets revealed the fact that Paip1 expression was augmented in tumor tissues in comparison with the normal cervical tissues (p < 0.01) (Fig. 1A and B). To verify of the informatic data, Paip1 expression in a group which consisted 130 cases of CC, 94 cases of CIN and 47 normal cervical tissues was figured out using IHC, and it was validated that Paip1 exhibited remarkable expression levels in CC tissues when compared with normal cervical epithelial tissues (Fig. 1C-H, S1 Fig.). Here, the positive rate of Paip1 expression was only 14.9% (7/47) in normal cervical epithelial tissues, but significantly higher in CIN lesions (50.00% in CIN-1, 58.8% in CIN-2, and 65.8% in CIN-3) and CC (79.2%, 90/174) (p < 0.05, respectively). Similarly, the strongly positive rate of Paip1 expression was observed in 55.4% (72/130) of CCs, which was significantly higher than in normal cervical epithelial tissues (4.3%, 2/47) (p < 0.01) (Table 1).
The correlations between Paip1 expression and clinicopathologic features were shown in Table 2. The high expression of Paip1 in CCs was significantly correlated with the clinical stage (p=0.019), differentiation (p=0.014), and lymph node metastasis (p=0.011) (Fig. 1I), but not with other factors included age or histological types. In all, these results indicated that high-level expression of Paip1 appears to be associated with CC progression.
Since we have confirmed the correlation between high Paip1 expression and CC progression, we next explored the potential roles of Paip1 in CC patients’ clinical outcome. By using The Human Protein Atlas, we showed that CC patients with high expression of Paip1 displayed a poor overall survival (OS) (Fig. 2A). Then Kaplan-Meier survival curves suggested that patients with high Paip1 expression had significantly shorter disease-free survival (DFS; p < 0.001) (Fig. 2B) and OS (p < 0.001) (Fig. 2C). Additionally, CC patients with overexpression of Paip1 had decreased DFS and OS compared to those with low Paip1 expression in either earlystage or late-stage cases (both p < 0.05) (Fig. 2D-G). Moreover, clinical stage, lymph node metastasis, and Paip1 expression status were correlated with shorter survival time in univariate analysis (Table 3). Multivariate Cox regression analysis further substantiated that Paip1 and clinical stage was an independent risk factor for lower DFS and shortened OS in patients with CC (Table 3). Based on the results above, we conclude that the overexpression of Paip1 can be regarded as a poor prognostic index in CC.
To investigate its potential function in CC progression, we firstly examined the expression of Paip1 in CC cell lines by western blot, and then stably knocked down Paip1 in high Paip1-expressing cells (SiHa and HeLa), and overexpressed Paip1 in low Paip1-expressing cell (C33A) (Fig. 3A). The expression levels of Paip1 in transfected cells were verified by western blot (Fig. 3B). Then, we assessed cell growth by MTT assays and found that CC cell lines with decreased Paip1 expression had slower growth rates, while CC cell lines with increased Paip1 expression had higher growth rates (Fig. 3C, S2A Fig.). Next, as determined by colony formation assays, Paip1 knockdown reduced the number of colonies formed, while Paip1 overexpression enhanced (Fig. 3D, S2B Fig. S3 Fig.). These results indicated that Paip1 promote the proliferation of CC cells in vitro.
For the purpose of decided on whether the impact of Paip1 on CC cell proliferation was the key observations of the Paip1-mediated alternations in the progression of the cell cycle, we carried out the flow cytometry assay in CC cell lines. As suggested by our observations, Paip1 knockdown gave rise to increased G2/M phase together with decreased G1 phase, as compared with the control cells (Fig. 4A). In addition, Paip1 knockdown altered the expression pattern of the key regulators that were indispensable in G2/M cell cycle regulation. Western blotting results indicated that cyclin B1 and CDK1 were decreased after Paip1 silencing, while cyclin-dependent kinase inhibitor P21, which was identified as tumor suppressor [17], was increased in the sh-Paip1 group (Fig. 4B, S2D-S2E Fig.). Conversely, the opposite effect was found in Paip1 overexpressed cells (Fig. 4A and B).
Subsequently, in order to study whether Paip1 can affect the apoptosis of CC cell lines, we carried out the flow cytometry assay in CC cell lines. As highlighted by the flow cytometry assay, Paip1 knockdown cells manifested higher apoptotic rate in comparison with the control group, whereas Paip1 overexpression displayed the opposite effect in CC cells (Fig. 5A, S2C Fig.). To further investigate which apoptosis pathway components were involved in CC cells response to Paip1 knockdown [18], we detected the expression of apoptosis-associated molecules by western blot. As shown in Fig. 5B, a marked increase in the pro-apoptotic Bax, and decrease in the anti-apoptotic Bcl-2 was observed after Paip1 knockdown. Meanwhile, the overexpression of Paip1 performed reserved trend (Fig. 5B). Collectively, Paip1 not only inhibited cell apoptosis and regulated cell cycle progression of CC cell lines, but also promote cell proliferation.
To further verify the cellular function of Paip1 in vivo, a xenograft mouse model was employed to study the role of Paip1 in CC growth. There was good agreement between in vivo and in vitro data. CC cells with stable knockdown or overexpression Paip1 were injected into nude mice, tumors derived from Paip1 knockdown group grew slower, and the average tumor weight was significantly lower. While tumor volume was markedly higher, and tumors were also heavier in mice which inoculated with Paip1 overexpressed cells (Fig. 6A and B, S4 Fig.). Next, we performed immunohistochemistry for Ki67, Bcl-2, and Bax in the xenografted tissues. As expected, knockdown Paip1 decreased the number Ki67 positive cells, reduced the expression of Bcl-2, and up-regulated the expression of Bax (Fig. 6C, S5 Fig.). The result was further confirmed in Paip1 overexpressed group (Fig. 6C, S5 Fig.). In conclusion, as an oncogene, Paip1 performs a substantial function in CC proliferation.
The regulation of gene expression occurs in the process of mRNA translation of eukaryotes, particularly at the translation initiation [19,20]. Deregulation at this step of the translation process results in abnormal expression of genes, which in turn changes cell growth and may lead to the development of cancer [21,22]. As a translational enhancer, Paip1 can stimulate translation rates in vivo, which have a promoter function in several tumors [11]. Besides, PAIP1 also plays a role in the decay of RNA. Grosset et al. [23] demonstrated that Paip1 was identified as one of the five mCRD-associated proteins. These proteins can form a multi-protein complex, stabilized mCRD-containing mRNA by impeding deadenylation. Nevertheless, the role of Paip1 in CC is still considered to be part of a growing sphere, which needs further investigation. Through this study, we identified for the first time the function of Paip1 in CC progression and the underlying mechanism.
We firstly studied the clinicopathological significance of Paip1 in CC, by performing immunohistochemistry. According to our results, Paip1 may have a tumor promoter function, and its expression could be a crucial prognostic indicator in CC. Our data showed that the expression of Paip1 was significantly elevated in CC tissues, while being absent in normal tissues. More importantly, the high Paip1 expression level was significantly associated with some clinicopathological parameters like clinical stage, differentiation, and lymph node metastasis. However, no differences were found with age or histological types. In particular, we found that CC patients exhibiting high Paip1 expression had poorer OS and DFS than patients with low expression. As a result, we believed that Paip1 plays a crucial role in patient prognosis, and the overexpression of Paip1 was an important characteristic in CC occurrence and progression.
Previous studies reported that Paip1 was overexpressed in breast cancer and can promote tumor-cell growth [13]. Consistent with these findings, our in vitro and in vivo studies have demonstrated a strong role of Paip1 in CC growth. Since cell cycle progression was a key step for tumor growth, and the aberrant cell cycle was closely related to the occurrence and development of cancers [24]. So, we assessed the change of cell cycle in CC cells transfect with or without Paip1, and found that Paip1 knockdown leads to G2/M cell cycle arrested, while Paip1 overexpression exhibited the opposite results. Moreover, we detected the change of cyclerelated proteins cyclin B1 and CDK1, which were responsible for the transition of the cell from the G2 to the M phase and their down-regulation would lead to G2/M cell cycle arrest [25]. As indicated by our data, Paip1 knockdown led to decreased expression of the cyclin B1 and CDK1, whereas Paip1 overexpression enhanced cyclin B1 and CDK1 expression. These results supported that Paip1 can control the progression of the cell cycle by regulating the expression of cell cycle proteins, thus affected CC cell proliferation.
Apoptosis was also a critical step for tumor development, and there were complex links between cell progression and cell apoptosis in the tumor. Bcl-2 and Bax were respectively anti-apoptotic and pro-apoptotic genes, and both of them belonged to Bcl-2 gene family [26]. Increased levels of proapoptotic proteins and/or decreased anti-apoptotic proteins can lead to apoptosis [27]. In the present report, Paip1 knockdown was found to induce apoptosis and followed a decrease of Bcl-2, an increase of Bax expression. Current thinking suggested that the ratio of Bax to Bcl-2 may play a vital role in cell apoptosis [28]. Thus, we conclude that Paip1 might be closely bonded with the apoptosis of CC cells through regulating the expression of apoptotic proteins, thereby influenced the proliferation of CC cell.
To summarize, as suggested by our research, Paip1 was overexpressed in CC samples and associated with the adverse outcomes in patients with CC, Paip1 knockdown induced inhibition of cell growth, G2/M arrest and apoptosis in CC cells (Fig. 7). These findings may provide the potential use of Paip1 as a therapeutic target of CC. Before that, a more comprehensive study was urgently needed to determine the mechanisms of Paip1 as an informative biomarker in patients with CC.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
ACKNOWLEDGMENTS
This study was supported by grants from National Natural Science Funds of China (NO. 31760313), The Funds of Changbai Mountain Scholar Project and Key Laboratory of the Science and Technology Department of Jilin Province (NO. 20170622007JC).
References
3. Kim JJ, Campos NG, Sy S, Burger EA, Cuzick J, Castle PE, et al. Inefficiencies and high-value improvements in U.S. cervical cancer screening practice: a cost-effectiveness analysis. Ann Intern Med. 2015; 163:589–97.
4. Kodama J, Seki N, Masahiro S, Kusumoto T, Nakamura K, Hongo A, et al. Prognostic factors in stage IB-IIB cervical adenocarcinoma patients treated with radical hysterectomy and pelvic lymphadenectomy. J Surg Oncol. 2010; 101:413–7.

5. Qiao L, Zheng J, Tian Y, Zhang Q, Wang X, Chen JJ, et al. Regulator of chromatin condensation 1 abrogates the G1 cell cycle checkpoint via Cdk1 in human papillomavirus E7-expressing epithelium and cervical cancer cells. Cell Death Dis. 2018; 9:583.

6. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.

7. Moore KN, Rowland MR. Treatment advances in locoregionally advanced and stage IVB/recurrent cervical cancer: can we agree that more is not always better? J Clin Oncol. 2015; 33:2125–8.

8. Martineau Y, Derry MC, Wang X, Yanagiya A, Berlanga JJ, Shyu AB, et al. Poly(A)-binding protein-interacting protein 1 binds to eukaryotic translation initiation factor 3 to stimulate translation. Mol Cell Biol. 2008; 28:6658–67.

9. Craig AW, Haghighat A, Yu AT, Sonenberg N. Interaction of polyadenylate-binding protein with the eIF4G homologue PAIP enhances translation. Nature. 1998; 392:520–3.

10. Kanaan AS, Frank F, Maedler-Kron C, Verma K, Sonenberg N, Nagar B. Crystallization and preliminary X-ray diffraction analysis of the middle domain of Paip1. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009; 65(Pt 10):1060–4.

11. Fukada Y, Yasui K, Kitayama M, Doi K, Nakano T, Watanabe Y, et al. Gene expression analysis of the murine model of amyotrophic lateral sclerosis: studies of the Leu126delTT mutation in SOD1. Brain Res. 2007; 1160:1–10.

12. Scotto L, Narayan G, Nandula SV, Subramaniyam S, Kaufmann AM, Wright JD, et al. Integrative genomics analysis of chromosome 5p gain in cervical cancer reveals target overexpressed genes, including Drosha. Mol Cancer. 2008; 7:58.

13. Piao J, Chen L, Jin T, Xu M, Quan C, Lin Z. Paip1 affects breast cancer cell growth and represents a novel prognostic biomarker. Hum Pathol. 2018; 73:33–40.

14. Yang Y, Wu Q, Li N, Che S, Jin T, Nan Y, et al. Upregulation of Tiam1 contributes to cervical cancer disease progression and indicates poor survival outcome. Hum Pathol. 2018; 75:179–88.

15. Chen L, Jin T, Zhu K, Piao Y, Quan T, Quan C, et al. PI3K/ mTOR dual inhibitor BEZ235 and histone deacetylase inhibitor Trichostatin A synergistically exert anti-tumor activity in breast cancer. Oncotarget. 2017; 8:11937–49.
16. Li N, Wang Y, Che S, Yang Y, Piao J, Liu S, et al. HBXIP over expression as an independent biomarker for cervical cancer. Exp Mol Pathol. 2017; 102:133–7.

17. Gao J, Yu H, Guo W, Kong Y, Gu L, Li Q, et al. The anticancer effects of ferulic acid is associated with induction of cell cycle arrest and autophagy in cervical cancer cells. Cancer Cell Int. 2018; 18:102.

18. Gorelick-Ashkenazi A, Weiss R, Sapozhnikov L, Florentin A, Tarayrah-Ibraheim L, Dweik D, et al. Caspases maintain tissue integrity by an apoptosis-independent inhibition of cell migration and invasion. Nat Commun. 2018; 9:2806.

19. Watkins SJ, Norbury CJ. Translation initiation and its deregulation during tumorigenesis. Br J Cancer. 2002; 86:1023–7.

20. Bitterman PB, Polunovsky VA. Translational control of cell fate: from integration of environmental signals to breaching anticancer defense. Cell Cycle. 2012; 11:1097–107.

21. Pestova TV, Kolupaeva VG. The roles of individual eukaryotic translation initiation factors in ribosomal scanning and initiation codon selection. Genes Dev. 2002; 16:2906–22.

22. Spilka R, Ernst C, Mehta AK, Haybaeck J. Eukaryotic translation initiation factors in cancer development and progression. Cancer Lett. 2013; 340:9–21.

23. Grosset C, Chen CY, Xu N, Sonenberg N, Jacquemin-Sablon H, Shyu AB. A mechanism for translationally coupled mRNA turnover: interaction between the poly(A) tail and a c-fos RNA coding determinant via a protein complex. Cell. 2000; 103:29–40.
24. Santo L, Siu KT, Raje N. Targeting cyclin-dependent kinases and cell cycle progression in human cancers. Semin Oncol. 2015; 42:788–800.

26. Lucantoni F, Lindner AU, O'Donovan N, Dussmann H, Prehn JH. Systems modeling accurately predicts responses to genotoxic agents and their synergism with BCL-2 inhibitors in triple negative breast cancer cells. Cell Death Dis. 2018; 9:42.

Fig. 1.
Poly(A)-binding protein-interacting protein 1 (Paip1) expression was significantly up-regulated in cervical cancer (CC). (A, B) Analysis of Paip1 expression in Oncomine data set. (C) Paip1 protein staining was negative in cervical epithelial tissues. (D, E) Paip1 protein staining was positive in dysplastic cells of cervical intraepithelial neoplasia. (F) Paip1 protein staining was positive in adenocarcinoma. (G, H) Paip1 protein staining was positive in squamous cervical cancers. (I) Relationship between Paip1 expression and clinicopathological significance of CCs. LN, lymph node.
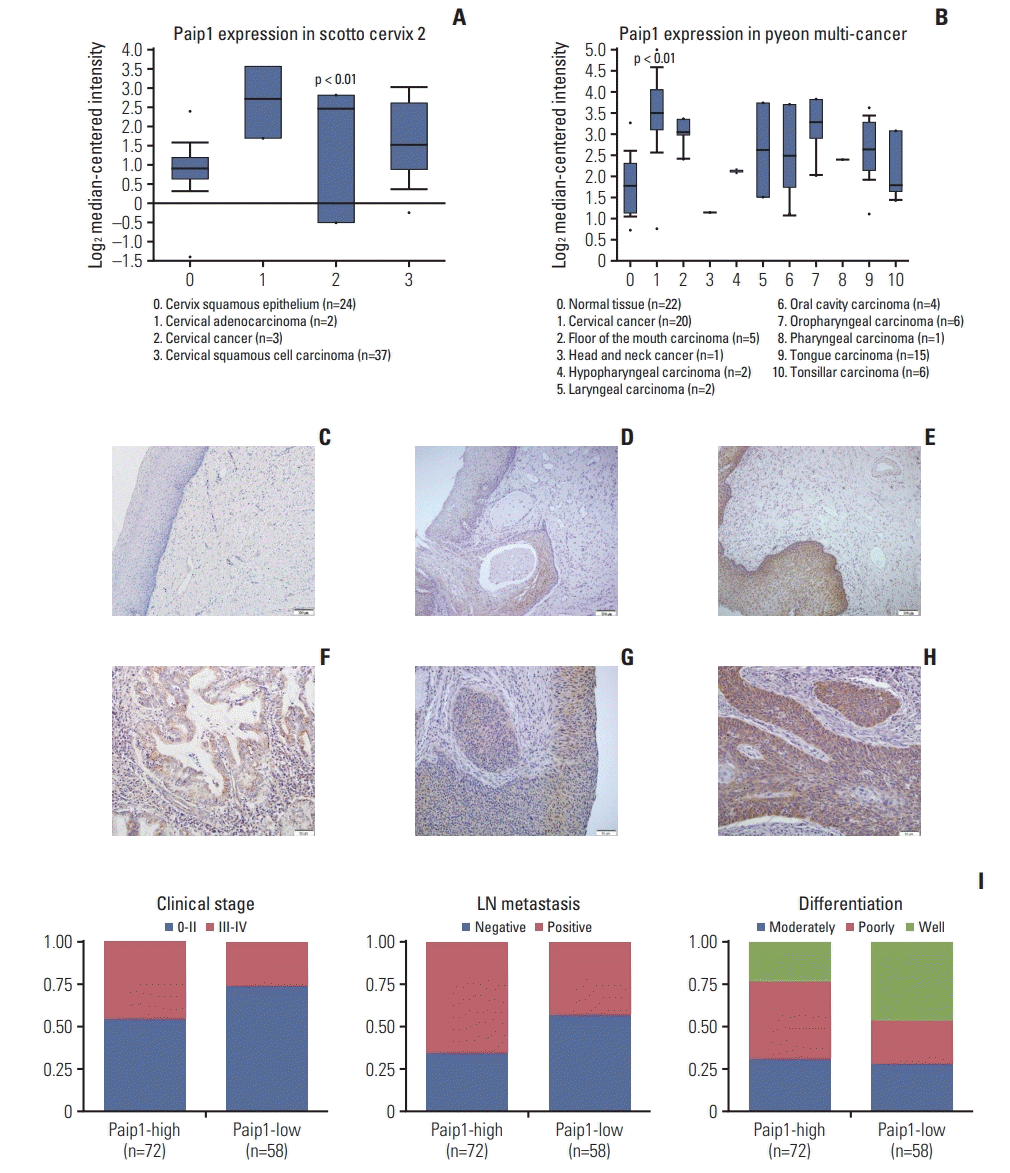
Fig. 2.
Poly(A)-binding protein-interacting protein 1 (Paip1) expression was associated with poor outcome in cervical cancer (CC). (A) The implication of Paip1 in the survival of patients with CC was determined in the Human Protein Atlas’s cohort. (B, C) Kaplan-Meier survival analyses were conducted to evaluate the influence of Paip1 on patient disease-free survival (DFS) (B) and overall survival (OS) (C). (D-G) Kaplan-Meier survival curves of CC patients in early and late stage.
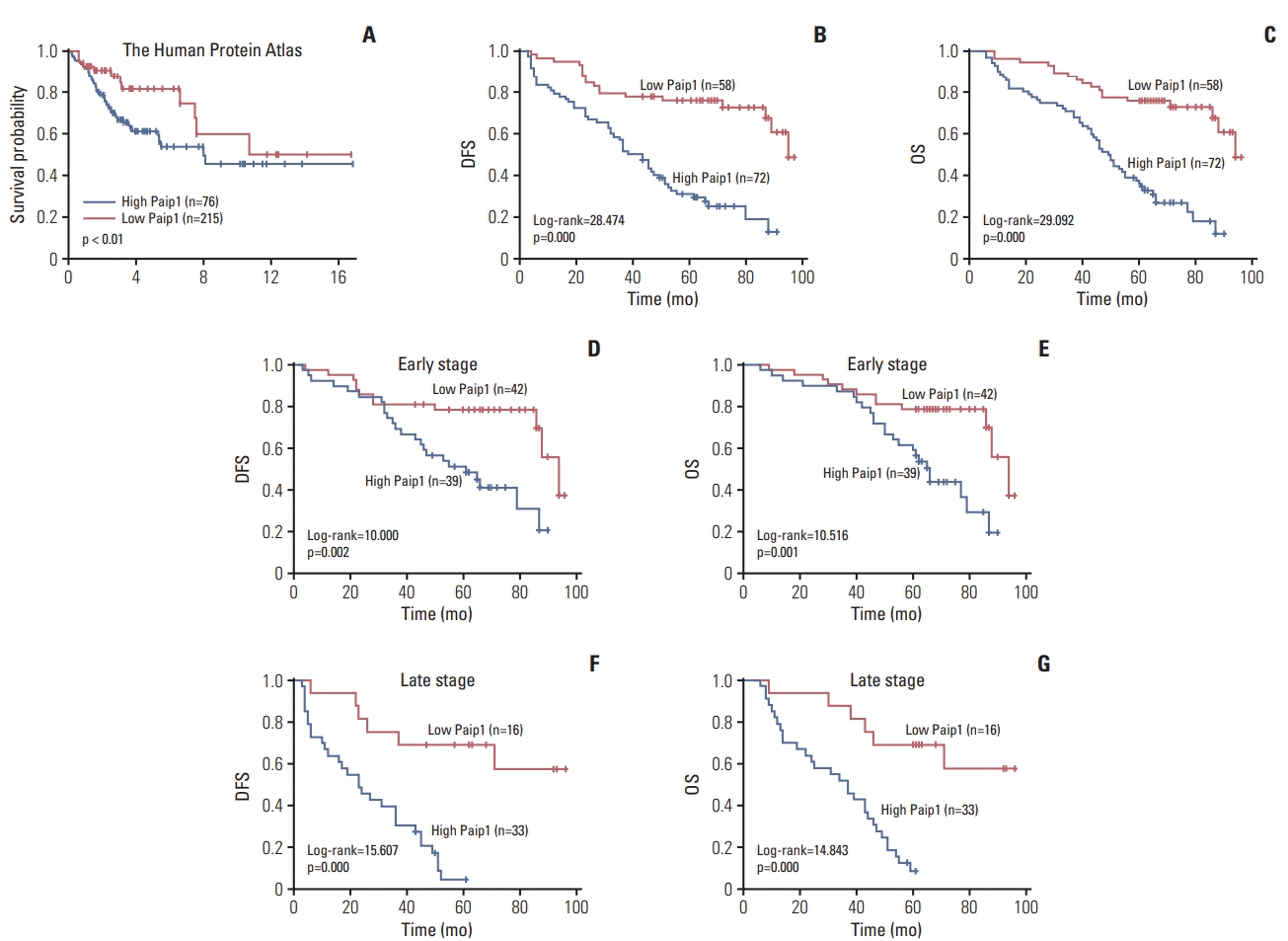
Fig. 3.
Poly(A)-binding protein-interacting protein 1 (Paip1) attenuated the ability of proliferation of cervical cancer (CC) cells in vitro. (A) The protein expression of Paip1 in CC cells was examined by western blot analysis. Actin was used as a loading control. (B) The protein expression of Paip1 in the constructed CC cells was confirmed by western blot analysis. Actin was used as a loading control. (C, D) Cell proliferation was examined by MTT (C) and colony formation (D) in the constructed cells. Data are represented as mean±standard error of mean of at least three independent experiments, **p < 0.01.
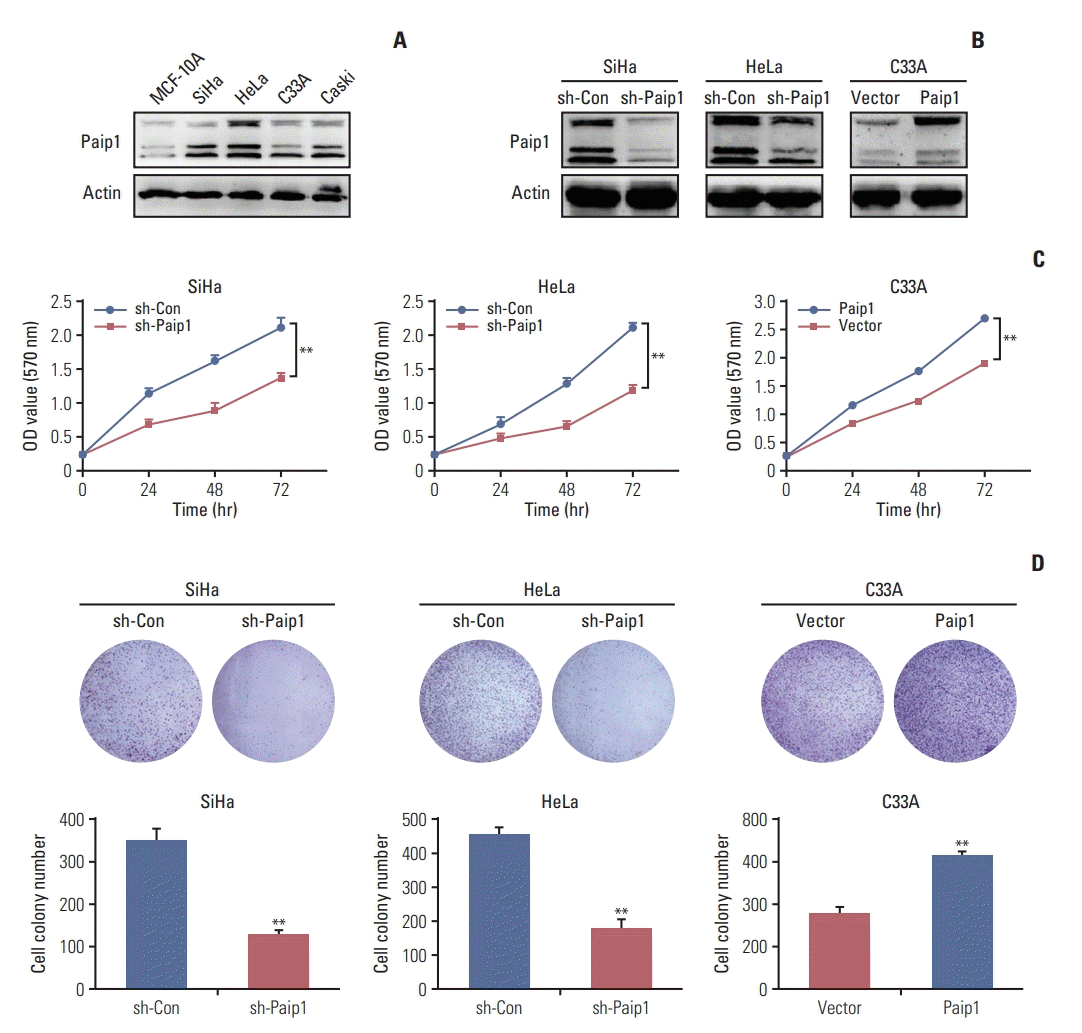
Fig. 4.
Poly(A)-binding protein-interacting protein 1 (Paip1) regulated cell cycle progression of cervical cancer (CC) cell lines in vitro. (A) Cell cycle distribution was identified by flow cytometry. Data are represented as mean±standard error of mean of at least three independent experiments. **p < 0.01. (B) The protein expression of molecules associated with the cell cycle in the constructed CC cells was confirmed by western blot analysis. Actin was used as a loading control.
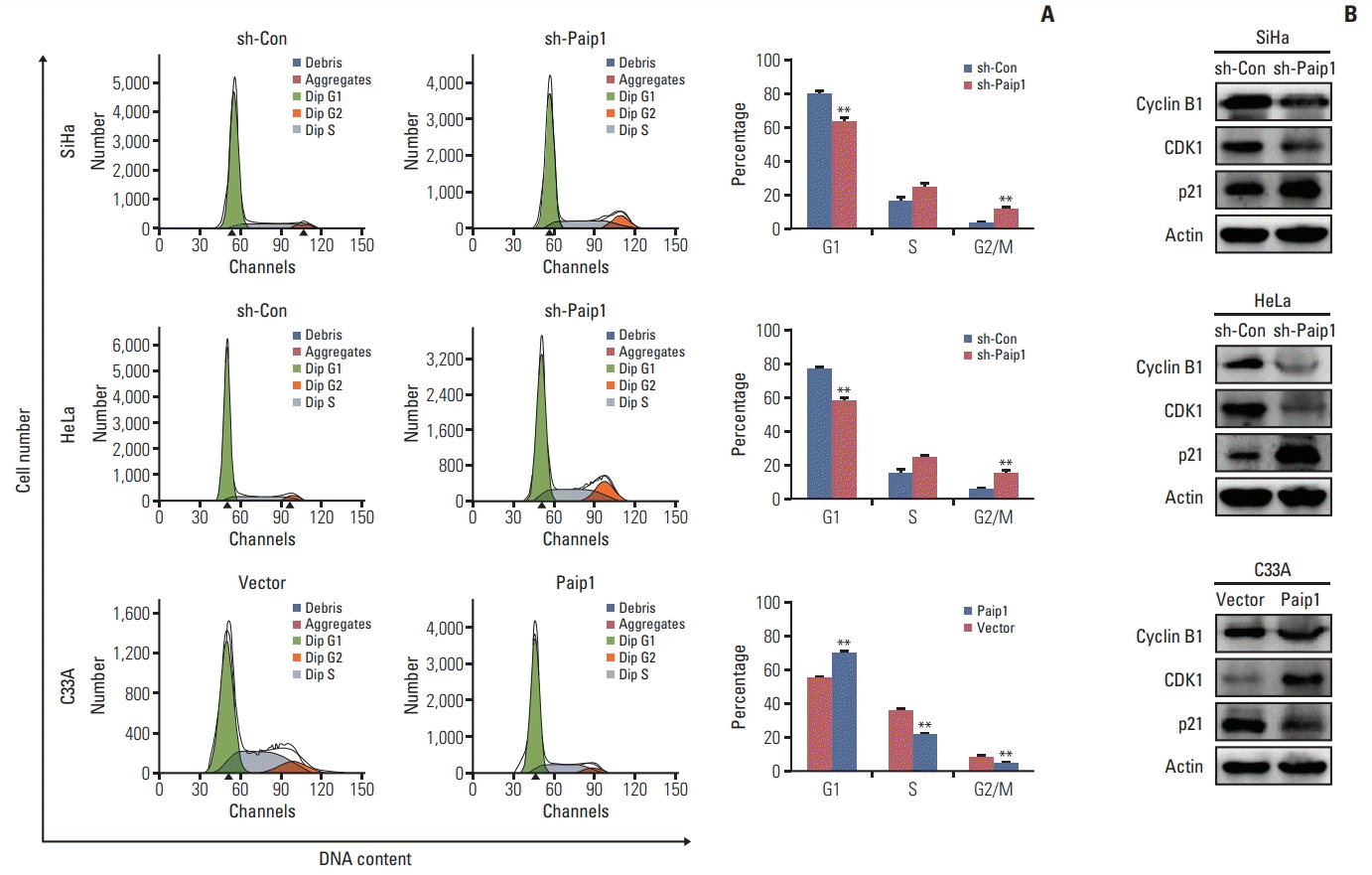
Fig. 5.
Poly(A)-binding protein-interacting protein 1 (Paip1) regulated cell apoptosis of cervical cancer (CC) cell lines in vitro. (A) Cell apoptosis of CC cells was detected by flow cytometry. Data were presented as the mean±standard deviation. **p < 0.01. (B) Western blot analysis of apoptosis-related protein in the constructed CC cells. Actin was used as a loading control. PI, propidium iodide.
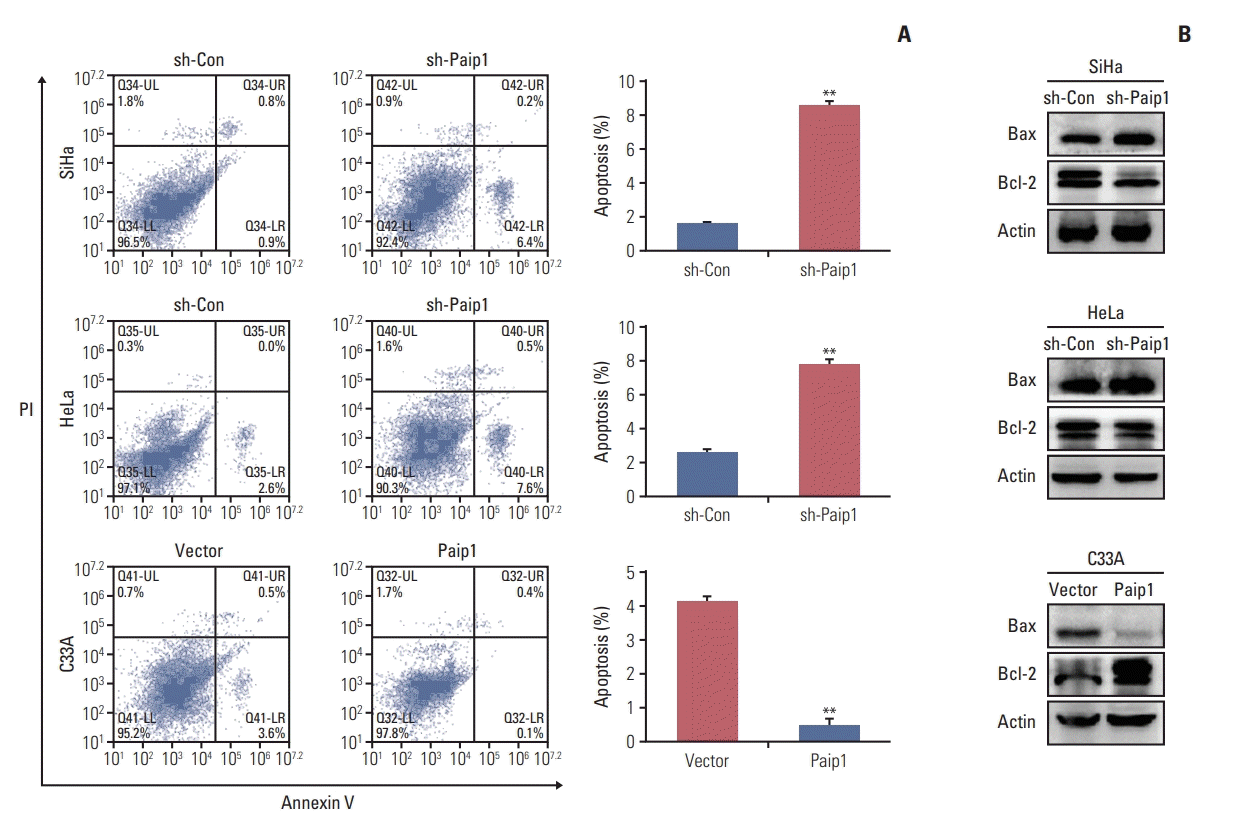
Fig. 6.
Poly(A)-binding protein-interacting protein 1 (Paip1) promoted tumor growth in vivo. (A, B) Indicated cells were injected into the nude mice, and statistical comparison of the differences in tumor weight was shown. (C, D) Expression levels of Ki-67, Bcl-2, and Bax in the xenograft tumors tissues were detected by immunohistochemistry.
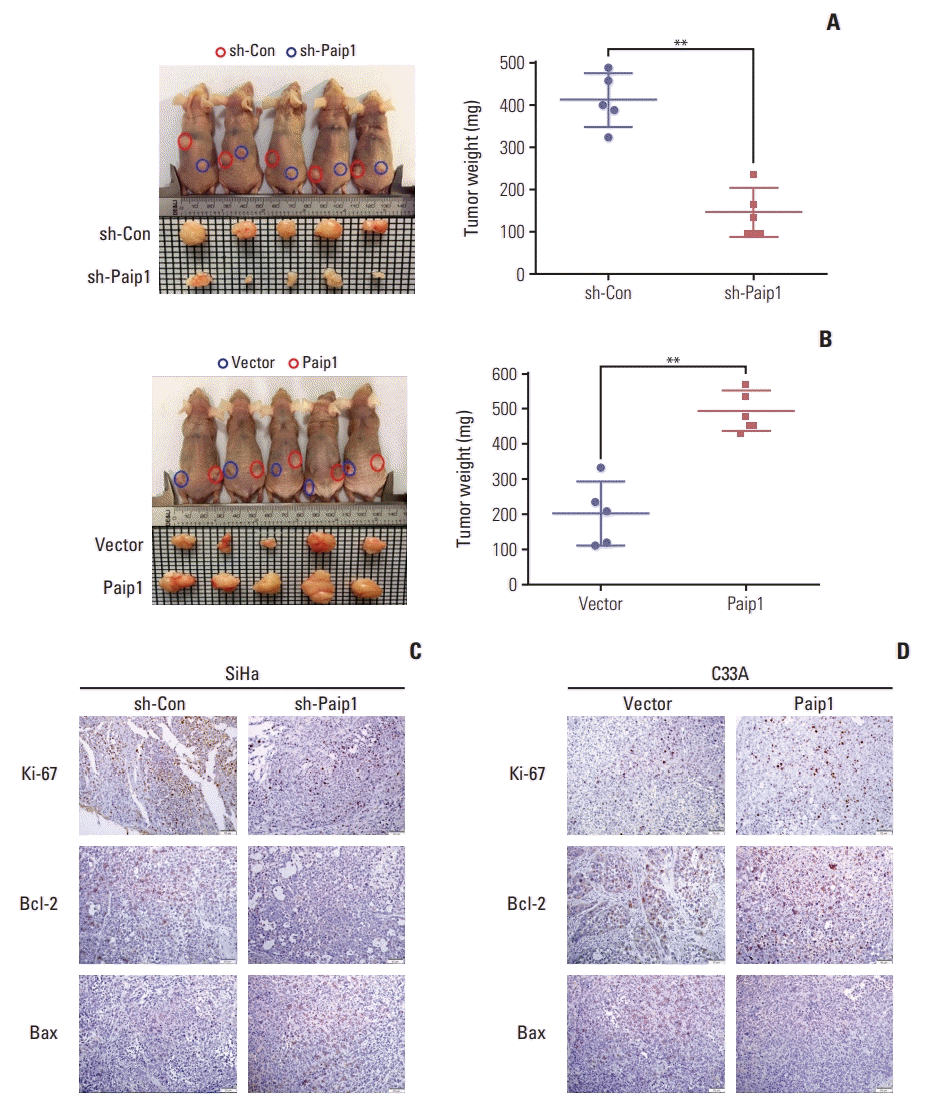
Fig. 7.
Schematic diagram. Poly(A)-binding protein-interacting protein 1 (Paip1) enhanced proliferation and inhibited apoptosis in cervical cancer.
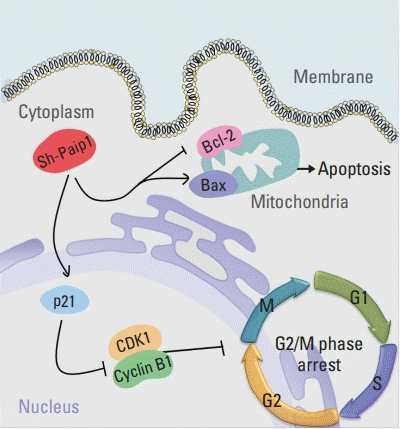
Table 1.
Paip1 protein expression in cervical cancers
Table 2.
Correlation between Paip1 expression and the clinicopathological features of cervical cancers
Table 3.
Cox regression model analysis of the clinicopathological features in 130 patients with cervical cancer




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download