Introduction
Materials and Methods
1. Human cell lines and reagents
2. Cell viability assay
3. Colony-forming assay
4. Western blot analysis
5. Cell cycle analysis
6. Alkaline comet assay
7. In vivo experiments
8. Immunohistochemistry
9. Statistical analysis
10. Ethical statement
Results
1. AZD6738 inhibits proliferation of BTC cells
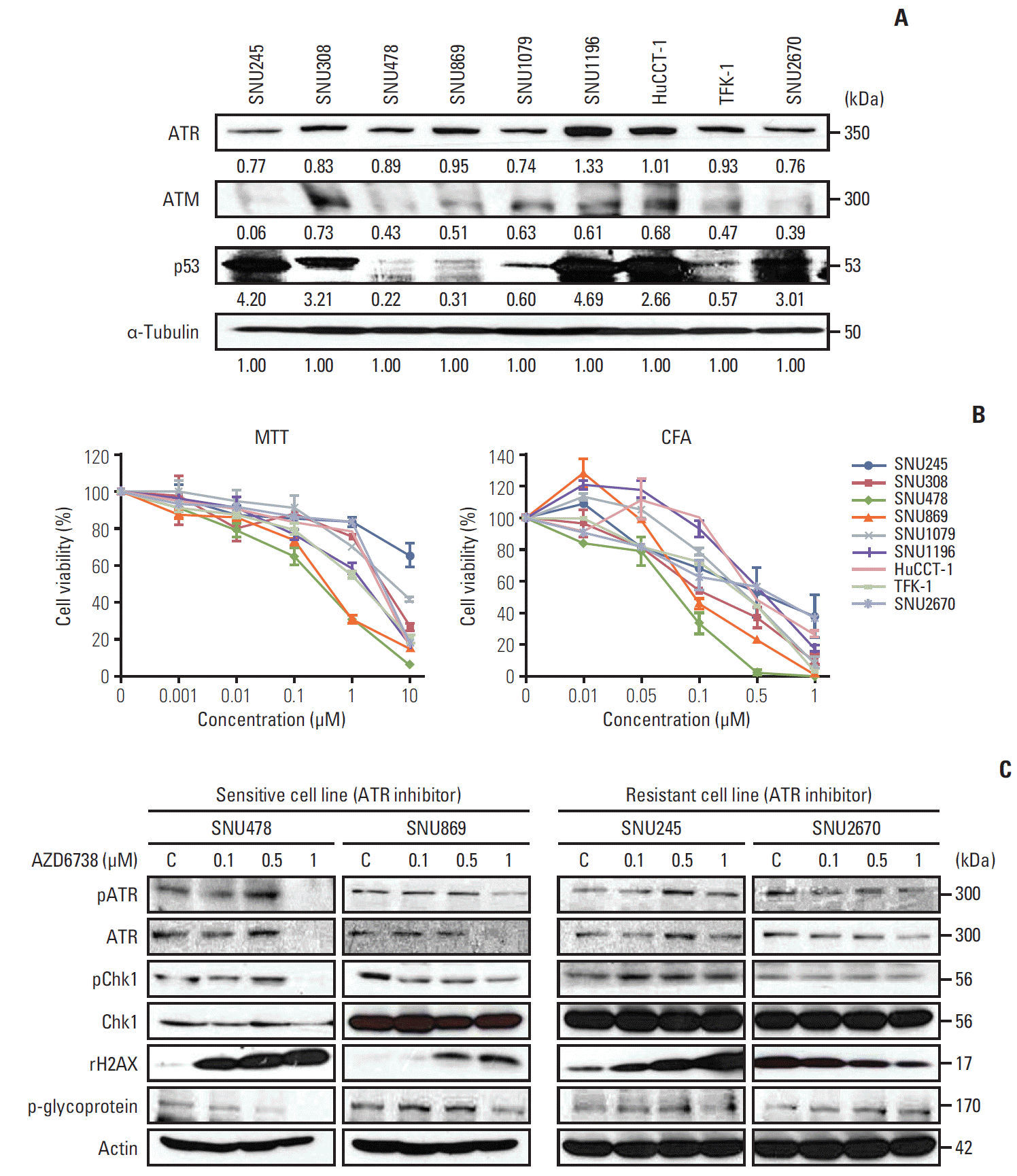 | Fig. 1.Anti-growth effect of AZD6738 in biliary tract cancer (BTC) cells. (A) Basal expression levels of ataxia-telangiectasia and Rad3-related (ATR), ataxia-telangiectasia mutated (ATM), and p53 in nine BTC cell lines analyzed by western blotting. The intensity was quantified using imageJ software. The intensity greater than “1” was classified as high expression, less than “0.6” was classified as low expression. (B) Anti-proliferative effects of AZD6738 in nine BTC cell lines evaluated by MTT assays (left) and colony-forming assays (CFA) (right). (C) Western blot analyses evaluating the effect of AZD6738 on signaling pathways in four BTC cell lines. SNU478, SNU869, SNU245, and SNU2670 cells were treated with increasing concentrations of AZD6738 (0, 0.1, 0.5, and 1 μM) for 5 days, after which protein extracts were immunoblotted with the indicated antibodies. |
2. AZD6738 leads to cell cycle arrest at sub-G1 and G2/M phases
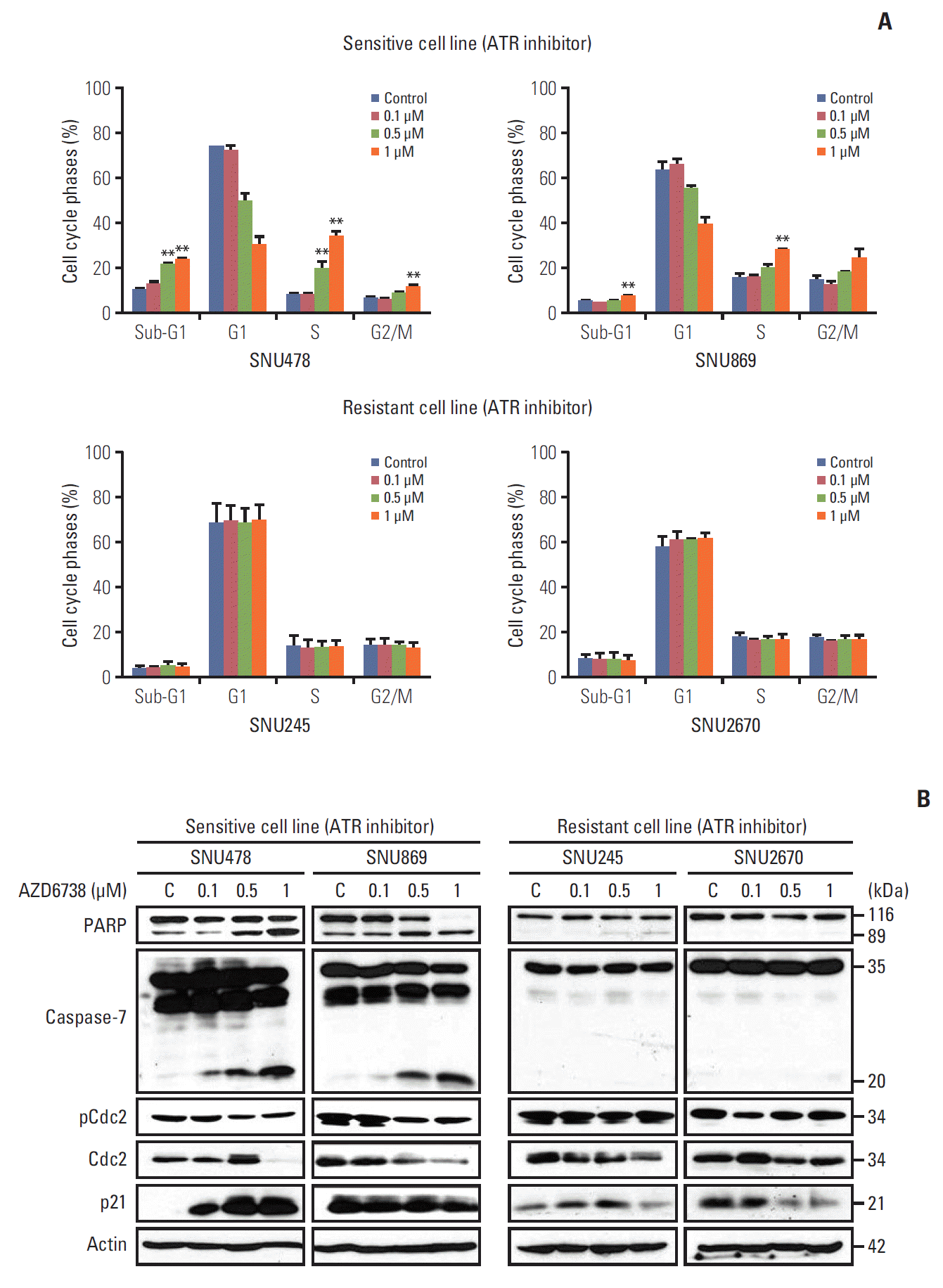 | Fig. 2.AZD6738 monotherapy induces cell cycle arrest in sensitive cell lines. (A) Cell cycle analyses of SNU478, SNU869, SNU245, and SNU2670 cells were performed by flow cytometry after treatment with increasing concentrations of AZD6738 (0, 0.1, 0.5, and 1 μM) for 3 days. The data represent three independent experiments. **p < 0.01. (B) Western blot analysis demonstrating the effect of AZD6738 on the expression levels of apoptosis and cell cycle checkpoint molecules in SNU478, SNU869, SNU245, and SNU2670 cells. The cells were treated with increasing doses of AZD6738 (0, 0.1, 0.5, and 1 μM) for 5 days. C, control. |
3. The synergistic anti-proliferative effects was observed by combined with AZD6738 and cytotoxic agents
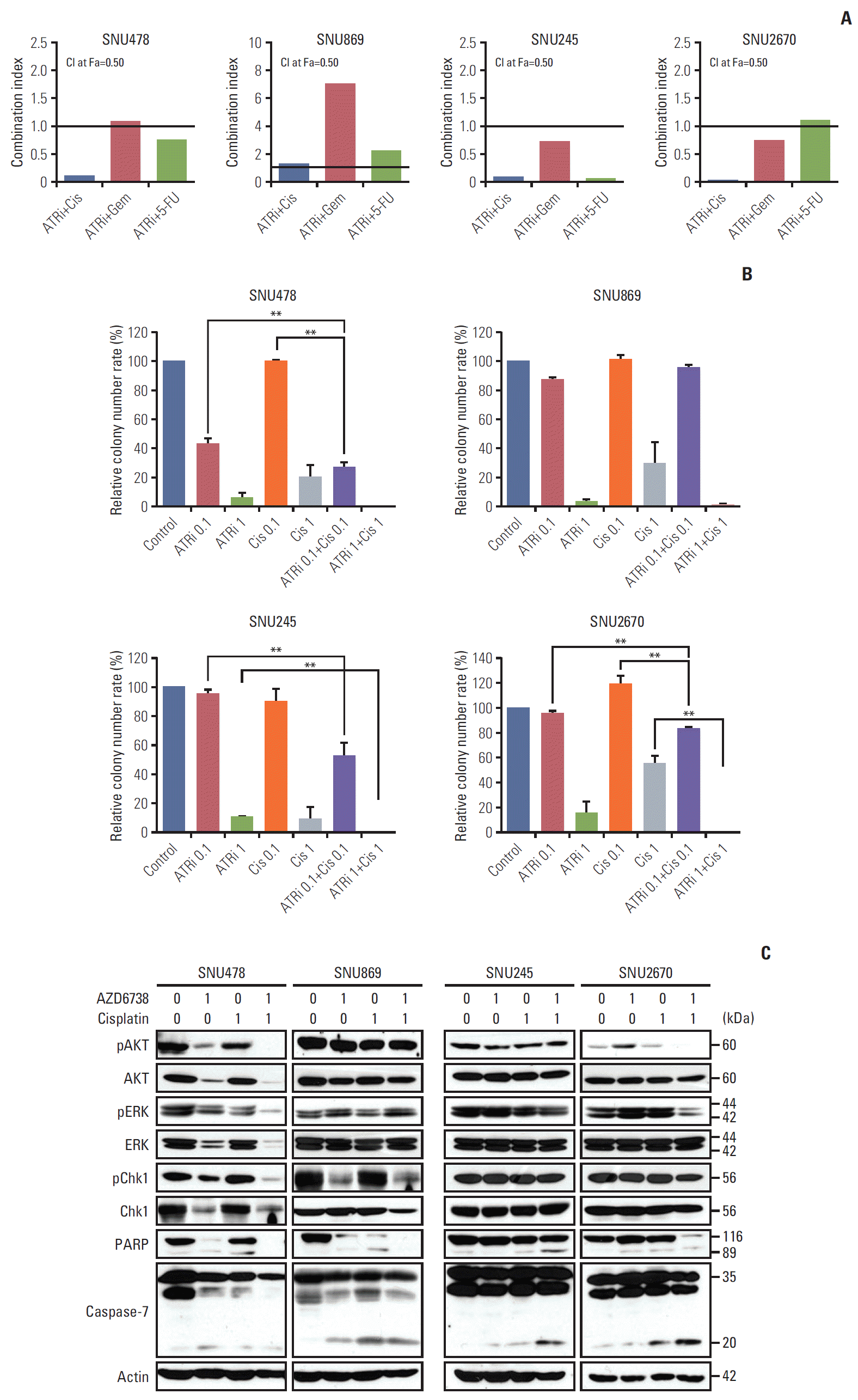 | Fig. 3.The synergistic effect was observed with combinations of AZD6738 and cytotoxic chemotherapeutic agents. (A) Anti-proliferative effects of combination chemotherapy of AZD6738 and cytotoxic agents (cisplatin, gemcitabine, and 5-fluorouracil [5-FU]) evaluated by MTT assays. The combination indexes for the combinations of AZD6738 and the cytotoxic agents at the ED50 were calculated by the Chou and Talalay method in the SNU478, SNU869, SNU245, and SNU2670 cell lines (combination index [CI] > 1, antagonistic effect; CI=1, additive effect; CI < 1, synergistic effect). (B) Colony-forming assays were performed to demonstrate the combined effect of AZD6738 and cisplatin. The concentration of each drug is indicated in the graph. The data represent mean±SE of three independent experiments. **p < 0.01. (C) Western blot analyses were performed to evaluate the anti-proliferative effect of AZD6738 and cisplatin on biliary tract cancer cell lines. PARP, poly(ADP-ribose) polymerase. |
4. Co-treatment of AZD6738 and cisplatin strongly induces DNA strand breaks
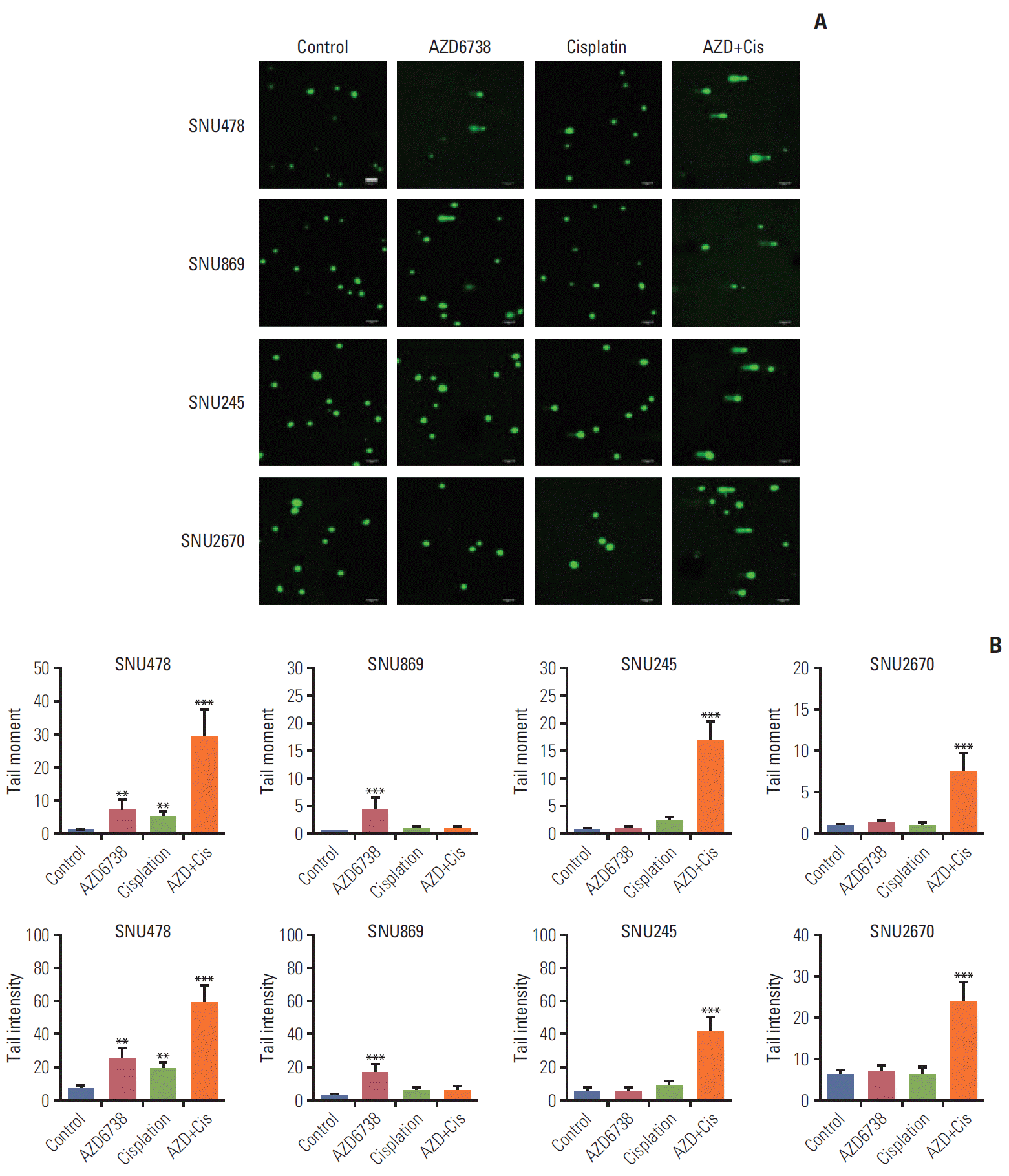 | Fig. 4.The potential impact of AZD6738 on DNA damage. (A) Comet assays were performed on SNU478, SNU869, SNU245, and SNU2670 cells treated with AZD6738 (0.5 μM), cisplatin (0.5 μM), or their combination for 5 days. Scale bars=100 μm. (B) Comparisons of comet tail length and tail intensity between cells treated with AZD6738 (0.5 μM), cisplatin (0.5 μM), or their combination for 5 days. The data represent mean±standard error of three independent experiments. **p < 0.01, ***p < 0.001. |
5. AZD6738 shows potent anti-tumor effects in vivo as a monotherapy and in combination with cisplatin
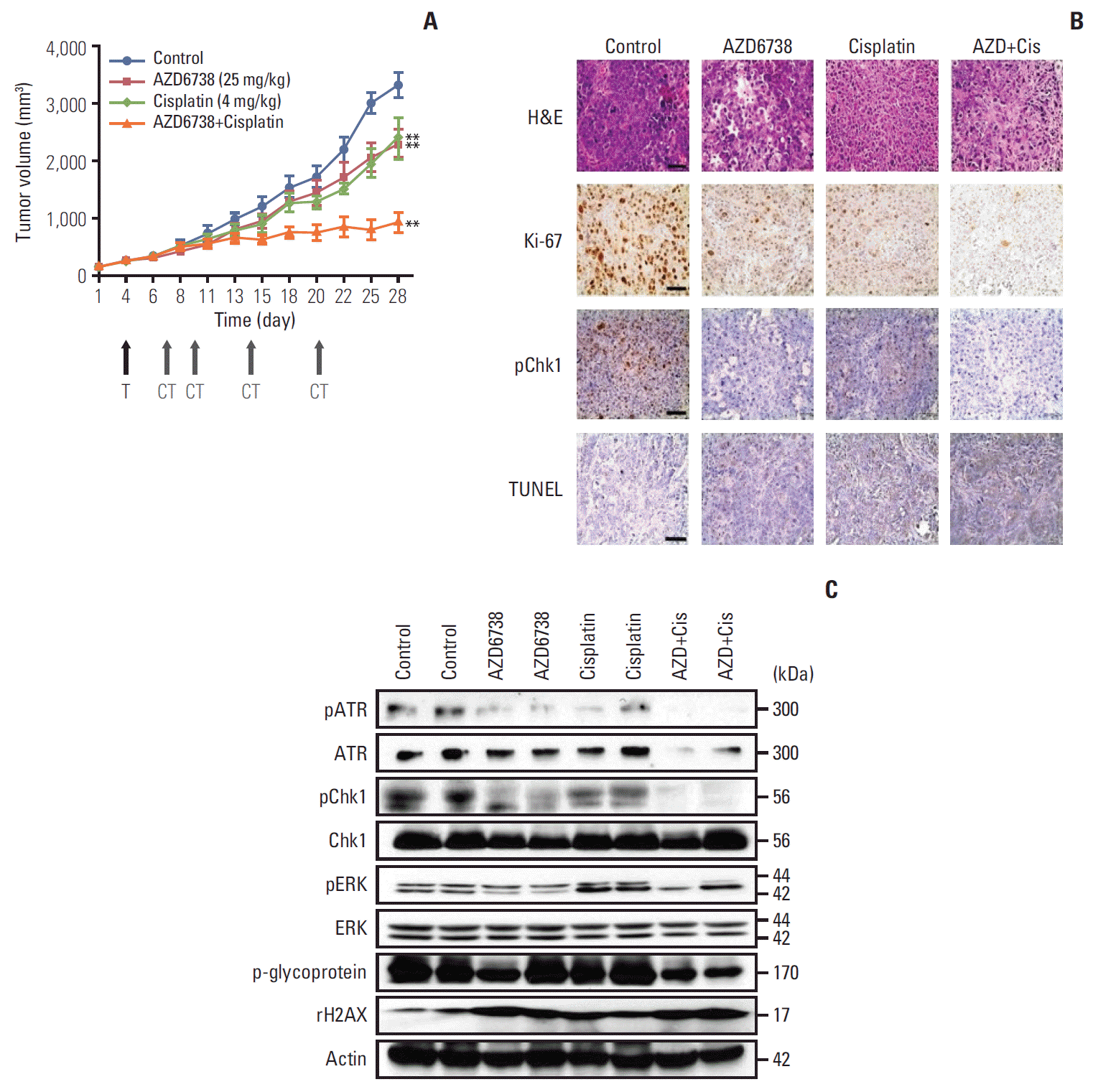 | Fig. 5.Anti-tumor growth of AZD6738 alone or combination therapy. (A) In vivo efficacy of AZD6738 (25 mg/kg), cisplatin (4 mg/kg), or their combination in SNU478 xenograft model mice. **p < 0.01. T, start of treatment; CT, day of cisplatin treatment. (B) The tumors were harvested and analyzed by immunohistochemistry. Ki-67, TUNEL expression, and Chk1 phosphorylation
were evaluated in the SNU478 xenograft model mice. Scale bars=50 μm. (C) Western blot assays were performed on the excised tumors from the xenograft model mice to elucidate the effects of AZD6738 and/or cisplatin. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download