Abstract
Purpose
We aimed to determine the demographic and epidemiologic variables that are associated with no treatment in lung cancer patients.
Materials and Methods
Patient data were collected from the Korean National Health Insurance Database. The lung cancer group included patients with an initial diagnosis of lung cancer between January 2009 and December 2014. Treated cases were defined as those that underwent surgery, radiation, or chemotherapy until death, after the diagnosis of lung cancer. Risk of no treatment was calculated by multiple logistic regression analysis.
Results
Among the 2,148 new cases of lung cancer from 2009 to 2104, 612 (28.4%) were not treated. Risk of no treatment was higher in the following patients: patients in their 60s (odds ratio [OR], 1.18; 95% confidence interval [CI], 0.75 to 1.84), 70s (OR, 3.64; 95% CI, 2.41 to 5.50), and >80 years old (OR, 16.55; 95% CI, 10.53 to 25.03) than those in their 50s; patients with previous myocardial infarction (OR, 2.07; 95% CI, 1.01 to 4.25) or chronic kidney disease (OR, 2.88; 95% CI, 1.57 to 5.30); and patients diagnosed at a non-referral hospital (OR, 1.40; 95% CI, 1.01 to 1.92) or primary care provider (OR, 1.81; 95% CI, 1.43 to 2.29) compared with referral hospital. Low-income patients receiving Medicaid were 1.75 times (95% CI, 1.14 to 2.68) more likely to forgo treatment than high-income patients (upper 20%). Risk was not associated with sex or the year in which the lung cancer was diagnosed.
Go to : 
Lung cancer is the most common cancer worldwide, ranking first in men and third in women for new cases, and it ranks first as the cause of death for both sexes [1]. Lung cancer is the third most common cancer in Korea, following stomach and colon cancers, and it is the most common cause of cancer deaths in Korea [2]. The 5-year survival rate of lung cancer in the United States is 18.6% while that for Korea is about 21.9% [3,4].
For a variety of reasons, some lung cancer patients do not undergo treatment. In the United States, untreated lung cancer patients were usually older than the treated group, more likely Black, uninsured, and of lower socioeconomic level [5]. Recent data from the United States indicate that 21% of all lung cancer patients did not receive anti-cancer treatment [6]. In Taiwan, 22.7% of patients with lung cancer are not treated [7]. In Korea, medical insurance is a part of social security with all citizens compulsorily enrolled in the government administered medical insurance. In addition, medical services are provided free of charge to citizens who earn below a certain income level. Therefore, it would be meaningful to find out the reason why cancer treatment is not received by citizens with access to free treatment under the national medical insurance system.
The development of new lung cancer therapies [8-12] and improved supportive care [13] have led to a steady improvement in lung cancer treatment, thus, not receiving treatment seems counterintuitive. It would be important to determine the characteristics of newly diagnosed lung cancer patients who did not receive treatment. This understanding will help in informing policies that can lower the barriers to receiving cancer treatment.
We aimed to identify demographic and epidemiologic variables associated with untreated lung cancer. We also compared the survival rates of treated and untreated lung cancer patients.
Go to : 
In Korea, there is only one health insurance system, with unique resident registration number for each citizen; thus avoiding duplication of subjects. The Korean National Health Insurance Service (KNHIS) covers more than 99% of all Korean residents and includes all health claim data such as diagnostic codes, procedures, prescription drugs, patient personal information, and hospital information.
This study used data from NHIS-NSC 2002-2015, which was released by the KNHIS in 2015, and includes all medical claims filed from January 2002 to December 2015 for 1,031,392 nationally representative randomly selected subjects, accounting for approximately 2.2% of the entire population in the KNHIS in 2002. The data were produced by the KNHIS using a systematic sampling method to generate a representative sample from all 46,605,433 Korean residents in 2002. The KNHIS data is linked to the Statistics Korea (national statistical office) data, which allows for accurate identification of deaths, by death certificate record.
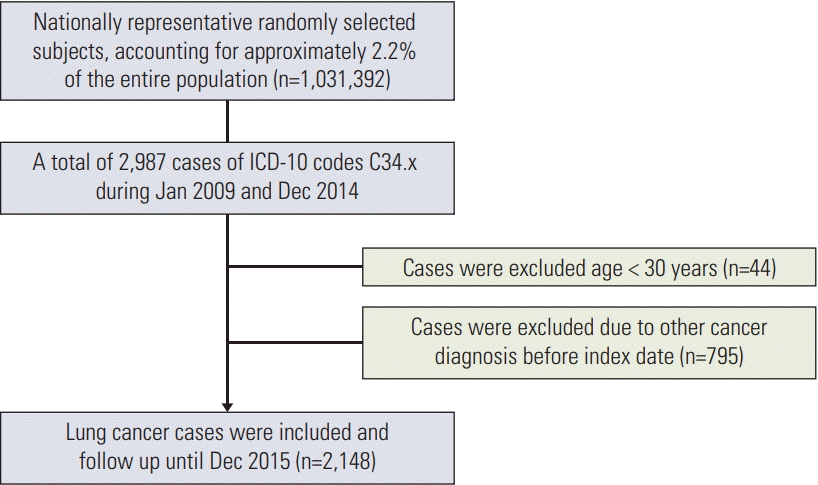
Patients with lung cancer between January 2009 and December 2014 were enrolled (Fig. 1). The International Classification of Diseases, 10th revision (ICD-10) codes were used as a key reference not only for disease diagnosis but also within the National Health Insurance (NHI) database.
The cases were followed up until December 2015. The diagnostic codes for lung cancer cases diagnosed before 2009 were maintained in the NHI database. New lung cancer cases were identified by counting new cases registered during the calendar year after excluding preexisting lung cancer. We counted newly developed lung cancer cases. Patients with lung cancer (C34) were included only if the patients had a special code, V193 or V194. Untreated cases were defined as those who had never undergone surgery, radiation, or chemotherapy until death, after diagnosis of lung cancer. The treated cases were defined by medical claim data.
The co-morbidities, diagnosed and identified using ICD-10 codes before the index date, included hypertension, diabetes mellitus, chronic kidney disease, myocardial infarction, venous thromboembolism, chronic obstructive pulmonary disease, and cerebrovascular accident.
Specific code for lung cancer (C34) has been implemented by the NHI service since 2009. All patients with lung cancer send documents to the NHI that align with their diagnosis. All patients with cancer are strictly validated and finally registered in a separate national cancer database maintained by the National Cancer Center (NCC). Patients whose cancer diagnoses were not validated were dropped off from the cancer code. Since 2009, patients classified as having cancer are eligible for payment reduction from the NHI; in line with the government’s improved policy regarding enhanced support for cancer patients. The physicians of such patients registered for lung cancer, sends the necessary eligibility documents to the NHI. This might have contributed to the successful implementation of this specific code for cancer in our health system.
Baseline characteristics at the initiation date (age, sex, residential area, household income, smoking status, income level, type of medical institution, and comorbidities) for cases and controls are summarized using descriptive statistics such as proportions. A chi-square test was used to compare frequencies of risk factors between untreated and treated groups. Logistic regression models were used to evaluate the risk factors for no treatment. The multivariate logistic regression models were constructed using patient age groups (30-49, 50-59, 60-69, 70-79, and ≥ 80 years), sex, household income (high, middle, low, very low, and Medicaid), geographic location (capital, large cities, and other), smoking status, facility type (referral hospital, non-referral hospital, and primary care provider), and co-morbidities. The Kaplan-Meier curve was used to calculate the 5-year survival rate between the non-treatment and treatment groups. p-values of < 0.05 were considered statistically significant. All statistical analyses were performed using SAS ver. 9.2 (SAS Institute, Cary, NC) and SPSS ver. 21 (IBM Corp., Armonk, NY).
The current study was approved by the institutional review board at Dongsan Medical Center, Keimyung University School of Medicine (IRB 2017-07-028) and written informed consent was waived.
Go to : 
Table 1 shows the baseline characteristics of the 2,148 patients in our study. Fifty-three percent (1,138 cases) of the patients, diagnosed of lung cancer, were above 70 years of age. The number of patients diagnosed at tertiary referral hospitals, at non-referral hospitals, and by primary care providers were 1,108 (51.6%), 748 (34.8%), and 292 (13.6%), respectively. Among the 2,148 new cases of lung cancer diagnosed between 2009 and 2104, 612 (28.4%) were not treated; and the older the patient, the higher the frequency of no treatment. Patients with no treatment were more likely to have chronic renal failure, hypertension, myocardial infarction, chronic obstructive pulmonary disease, or cerebrovascular accident. The residential area in which the cancer was first diagnosed differed significantly between the treatment and no treatment groups. There was no difference in percentage of lung cancer treatment between calendar year.
Demographic and clinical characteristics of patients with lung cancer untreated and treated
Multivariate logistic regression analysis of the variables (Table 2) revealed a significant association with no treatment; with the lowest income group (Medicaid) having 1.7 times the risk, history of myocardial infarction had 2.0 times, history of chronic kidney disease about 2.8 times, and most notably, the age. Relative to patients in their 50s, risk of nontreatment was 1.1 times higher in patients in their 60s, 3.6 times higher in patients in their 70s, with a sharp increase to 16.5 times in patients > 80 years of age. Lung cancer diagnosis at a non-referral hospital or by a primary care provider significantly increased risk of no treatment compared to referral hospital. All risk factors included in the model had relatively narrow confidence intervals. There were no variables that changed the direction of the odds ratio in the univariate and multivariate analyses except income and year of diagnosis, which was statistically not significant. The magnitude of the odds ratios of previous history of myocardial infarction and chronic kidney disease was reduced after the multivariate analysis. However, age at lung cancer diagnosis showed almost the same odds ratio at multivariate analysis.
Univariate and multivariate logistic regression analyses for factors associated with the no treatment of lung cancer
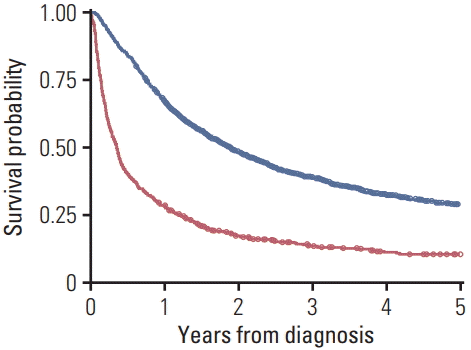
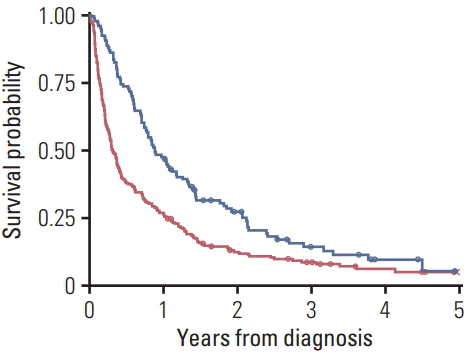
Five-year survival rate was lower in the no treatment vs. treatment group (Fig. 2). The median survival time was 18.1 months longer in the treatment group. When only patients > 80 years old were analyzed, 5-year survival rate was lower in the no treatment group, which also had a shorter median survival time (by 6.8 months) (Fig. 3).
Go to : 
In this study, 31% of lung cancer patients in their 70s and 67% of those > 80 years of age did not receive treatment. The percentage of those > 80 years of age in our study that did not receive treatment, is higher than that in a study in the United States (about 50%) [14]. In our study, when other variables were controlled for, patients in their 70s were 3.6 times more likely to decline being treated than those in their 50s, and patients > 80 years old were 16.5 times more likely to decline being treated. In Taiwan, the odds ratio for declining treatment for lung cancer was 2.61 in patients > 75 years old compared to patients ≤ 44 years old, as determined in analyses controlled for confounders and comorbidities. Hence, there is a large difference in the percentage of untreated lung cancer patients in Korea and Taiwan, despite their similar cultural, ethnic, and economic backgrounds [7].
When the physicians are planning to assign specific codes (V193 or V194) for lung cancer, they were to submit the exact diagnostic method to the national health insurance. In this study, lung cancer was defined using both the specific code and the ICD-10 code and this might have resulted in the selection of patients with lung cancer, using the two codes; this might have resulted in an overestimation. However, the number of lung cancer patients from the National Cancer Register and in this study, is consistent. The number of lung cancer patients aged 30 years or older included in the National Cancer Register during the same period of this study was 133,503 [15]. The database (NHIS-NSC 2002-2015) used in this study, was based on 2.2% of the whole national population. Therefore 2,937 cases of lung cancer should occur in this database if the incidence of lung cancer is the same as the National Cancer Register. This study excluded patients (n=795) who had cancer before the diagnosis of lung cancer (Fig. 1). Therefore, 2,142 patients with lung cancer would occur during the study period, excluding patients who had previously had cancers.
Staging would be an important factor for determining anticancer treatment in lung cancer patients. However, in this study, staging of the treated and the non-treated group was not available. Our study needs to validate considering staging of all lung cancer patients, while retrospective study failed to demonstrate significant association between lung cancer treatment refusal and staging in Korea [16].
In Korea, life expectancy is 78.8 years for men and 85.5 years for women [17]. Hence, Korean cancer patients in their 80s may forgo treatment thinking they have reached the end of their lives; physical and social barriers may also play a role. These barriers among elderly lung cancer patients need to be overcome [18,19].
The likelihood of refusing treatment was 2.4% higher in women than men in the U.S. study [6] and 2% higher in the Taiwan study [7]. In our study, it was 3% higher, although this increase was not statistically significant. Because men have a shorter average lifespan than women, they were more likely to receive help from their spouses during their treatment, while women were less likely to [20].
As income decreases, the risk of not receiving cancer treatment increases. In this study, the lowest income group (Medicaid) was 1.7 times less likely to undergo treatment than the highest income group (top 20%). Previous studies reported a similar finding, although the difference between the income groups was smaller [6,7]. Regardless of income, > 99% of Korean citizens are covered by the health insurance. Individuals with the lowest income are supported by the government with almost all medical expenses including medications paid for. However, this does not offset the effect of economic factors on treatment received in Korea, like in other countries [21]. For the lowest income group, additional social care may be needed in addition to the insurance benefit [22].
In this study, not receiving treatment for lung cancer significantly correlated with the history of myocardial infarction or chronic kidney disease. Co-morbidities were also associated with no treatment in previous studies of lung and ovarian cancer patients [6,7,23], whereas another study found no association [24]. Age was an important determinant of treatment refusal in our study, as well as in other studies [14]. Educating otherwise healthy elderly patients with lung cancer, and supporting their families may help persuade them to accept treatment [25,26].
Physician preferences regarding referrals have been shown to influence treatment vs. no treatment decisions [27]. In our study, lung cancer patients diagnosed at non-referral hospitals or by primary care providers were, respectively, 1.4 and 1.8 times less likely to receive treatment than those diagnosed at referral hospitals (Table 2). Information about lung cancer needs to be provided, not only to patients’ families but also, to medical personnel that are not specialized in lung cancer [28]. Active medical policies, medical institutions, and community efforts are also required to ensure that cancer treatment is acceptable to all cancer patients [29].
Unlike a previous study in the United States [6], our study showed that risk of no treatment was stable over time when other variables were controlled (Table 2). In Korea, the number of treatment items covered by insurance increases yearly, and the number of patients receiving treatment is expected to increase owing to decreases in the costs borne by patients. This may partially explain the difference between our results and those from the United States [6].
Lastly, we found that the survival rate was significantly higher in the treatment group than the no treatment group, even when only patients > 80 years old were considered. The median survival time was 6 months longer in treated vs. untreated for the “over 80” patients. This suggested that active treatment could be an important option even for those aged > 80 years in selected lung cancer patients [30]. Although we did not consider confounders, co-morbidities, and treatment complications in the survival data, the data are presented for comparison with other similar studies.
The limitations of current study are the following: we did not have data on stages of lung cancer, which should be an important factor in deciding the treatment plan. And there was no information on the patient’s personal reasons for no treatment, as well as about the family’s attitudes toward forgoing the treatment. There was also no information about the alternative care of the untreated lung cancer patients. In addition, diagnoses of comorbidities were based on the ICD codes, which may require validation via medical record review.
Although economic status, non-referral hospital, renal failure, and history of myocardial infarction were important risk factors that are associated with no treatment in lung cancer, age predominantly determined untreated lung cancer. Full support of medical expense in the lowest income group does not reduce untreated lung cancer patients. Survival rates and times were higher in treated vs. untreated patients, even among those > 80 years of age.
Lung cancer treatments continue to evolve in ways that produce better responses with less toxicity. The aging of society is a worldwide phenomenon, with some countries having “super-aged” populations, and the incidence of lung cancer in the elderly is increasing. Therefore, more studies are needed to investigate the age-related treatment barriers to lung cancer treatment.
Go to : 
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIP) (No. 2014R1A5A2010008).
Go to : 
References
1. Thun MJ, Henley SJ, Travis WD. Lung cancer. In : Thun MJ, Linet MS, Cerhan JR, Haiman CA, Schottenfeld D, editors. Cancer epidemiology and prevention. New York: Oxford University Press;2018. p. 519–41.
2. Jung KW, Won YJ, Kong HJ, Lee ES; Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2015. Cancer Res Treat. 2018; 50:303–16.

3. National Cancer Institute Surveillance Epidemiology, and End Results Program. SEER Cancer Stat Facts: Lung and Bronchus Cancer [Internet]. Bethesda, MD: National Cancer Institute;c2018. [cited 2018 Aug 6]. Available from: http://seer.cancer.gov/statfacts/html/lungb.html.
4. Park JY, Jang SH. Epidemiology of lung cancer in Korea: recent trends. Tuberc Respir Dis. 2016; 79:58–69.

5. Small AC, Tsao CK, Moshier EL, Gartrell BA, Wisnivesky JP, Godbold JH, et al. Prevalence and characteristics of patients with metastatic cancer who receive no anticancer therapy. Cancer. 2012; 118:5947–54.

6. David EA, Daly ME, Li CS, Chiu CL, Cooke DT, Brown LM, et al. Increasing rates of no treatment in advanced-stage nonsmall cell lung cancer patients: a propensity-matched analysis. J Thorac Oncol. 2017; 12:437–45.
7. Huang HL, Kung PT, Chiu CF, Wang YH, Tsai WC. Factors associated with lung cancer patients refusing treatment and their survival: a national cohort study under a universal health insurance in Taiwan. PLoS One. 2014; 9:e101731.

8. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–57.

9. Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in nonsmall-cell lung cancer. N Engl J Med. 2010; 363:1693–703.
10. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015; 373:1627–39.

11. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015; 372:2018–28.

12. Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, et al. AZD9291 in EGFR inhibitor-resistant nonsmall-cell lung cancer. N Engl J Med. 2015; 372:1689–99.

13. Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010; 363:733–42.

14. Wang S, Wong ML, Hamilton N, Davoren JB, Jahan TM, Walter LC. Impact of age and comorbidity on non-small-cell lung cancer treatment in older veterans. J Clin Oncol. 2012; 30:1447–55.

15. Korean Statistical Information Service. Cancer incidence in Korea [Internet]. Daejeon: Statistics Korea;2016. [cited 2018 Nov 3]. Available from: http://kosis.kr/statHtml/statHtml.do?orgId=117&tblId=DT_117N_A0024&conn_path=I3.
16. Suh WN, Kong KA, Han Y, Kim SJ, Lee SH, Ryu YJ, et al. Risk factors associated with treatment refusal in lung cancer. Thorac Cancer. 2017; 8:443–50.

17. World Health Organization. Life expectancy increased by 5 years since 2000, but health inequalities persist [Internet]. Geneva: World Health Organization;c2016. [cited 2018 Aug 7]. Available from: http://www.who.int/en/news-room/detail/19-05-2016-life-expectancy-increased-by-5-years-since-2000-but-health-inequalities-persist.
18. Shingler SL, Bennett BM, Cramer JA, Towse A, Twelves C, Lloyd AJ. Treatment preference, adherence and outcomes in patients with cancer: literature review and development of a theoretical model. Curr Med Res Opin. 2014; 30:2329–41.

19. van Kleffens T, van Leeuwen E. Physicians' evaluations of patients' decisions to refuse oncological treatment. J Med Ethics. 2005; 31:131–6.

20. Puts MT, Monette J, Girre V, Wolfson C, Monette M, Batist G, et al. Characteristics of older newly diagnosed cancer patients refusing cancer treatments. Support Care Cancer. 2010; 18:969–74.

21. Lin JH, Zhang SM, Manson JE. Predicting adherence to tamoxifen for breast cancer adjuvant therapy and prevention. Cancer Prev Res (Phila). 2011; 4:1360–5.
22. Min HS, Yang HK, Park K. Supporting low-income cancer patients: recommendations for the public financial aid program in the Republic of Korea. Cancer Res Treat. 2018; 50:1074–83.

23. Wallace SK, Lin JF, Cliby WA, Leiserowitz GS, Tergas AI, Bristow RE. Refusal of recommended chemotherapy for ovarian cancer: risk factors and outcomes; a national cancer data base study. J Natl Compr Canc Netw. 2016; 14:539–50.

24. Janssen-Heijnen ML, Smulders S, Lemmens VE, Smeenk FW, van Geffen HJ, Coebergh JW. Effect of comorbidity on the treatment and prognosis of elderly patients with non-small cell lung cancer. Thorax. 2004; 59:602–7.

25. Marshall CA, Badger TA, Curran MA, Koerner SS, Larkey LK, Weihs KL, et al. Un Abrazo Para La Familia: providing lowincome Hispanics with education and skills in coping with breast cancer and caregiving. Psychooncology. 2013; 22:470–4.

26. Turner D, Adams E, Boulton M, Harrison S, Khan N, Rose P, et al. Partners and close family members of long-term cancer survivors: health status, psychosocial well-being and unmet supportive care needs. Psychooncology. 2013; 22:12–9.

27. Goulart BH, Reyes CM, Fedorenko CR, Mummy DG, Satram-Hoang S, Koepl LM, et al. Referral and treatment patterns among patients with stages III and IV non-small-cell lung cancer. J Oncol Pract. 2013; 9:42–50.

28. Murgu S, Rabito R, Lasko G, Jackson C, Mino-Kenudson M, Ettinger DS, et al. Impact of a non-small cell lung cancer educational program for interdisciplinary teams. Chest. 2018; 153:876–87.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download